Chapter 16: MOA Part 2: Amino Acid Synthesis Inhibitors & Nitrogen Metabolism Inhibitors
16.3 Branched Chain Amino Acid Biosynthesis and the ALS Enzyme
Let us now focus our attention on the first herbicide Site of Action within the Amino Acid Synthesis MOA, the ALS Inhibitors, starting with a discussion on branched chain amino acids.
Branched chain amino acid production is important for several reasons. Valine, leucine and isoleucine represent 3 of the 10 essential amino acids needed for human nutrition. These amino acids are critical for protein synthesis and normal plant growth, while also providing precursors for a number of secondary metabolites such as; cyanogenic glycosides, glucosinolates, and acyl-sugars. Branched chain amino acid production also represents a commercially important target for a variety of low-use-rate herbicides.
Branched chain amino acid production starts with two different substrates. In the biochemical pathway diagramed here, we see that on the left side, valine and leucine production starts with pyruvate derived directly from photosynthesis. On the right side, we find that isoleucine production starts with threonine (an amino acid). There are three enzymes shared in the production of all three amino acids, but for purposes of our discussion, we will focus only on the first shared enzyme, acetolactate synthase (ALS).
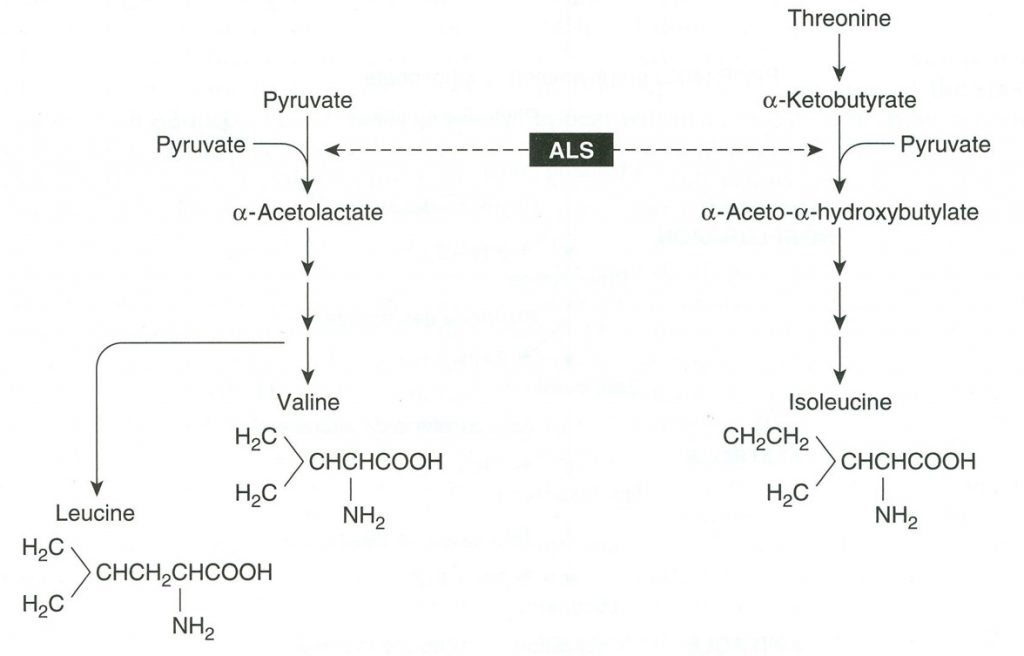
The gene that encodes for ALS is found in the plant cell nucleus; however, the pathway leading to the formation of the amino acids is located in the chloroplast. That means the ALS enzyme must be transported from the nucleus to the chloroplast. Since the major precursor needed to start the branched chain amino acids biosynthesis is a product of photosynthesis it makes sense energetically to transport the enzyme to the chloroplast. This is accomplished in the same manner as with EPSP synthase (discussed later in this chapter). The gene encoding ALS has a “leader sequence” that directs the enzyme to the chloroplast where it is cleaved to produce an active ALS enzyme.