Main Body
Amyloidosis
- Amyloid is the general term used for a wide variety of extracellular insoluble protein accumulations made of fibrils that have a β-pleated structure.
- In the kidney, amyloid is predominantly located in the glomeruli with fewer cases having interstitial or vascular amyloidosis.
- Histologic appearance – Nodular expansion of the mesangium and capillary walls by congophilic material (from Congo red [CR] staining) that is peach to orange. This material exhibits apple-green birefringence when viewed with polarized light.
- Amyloid appears pale pink and waxy when stained with PAS, mottled blue to orange with MT and does not take up silver with the JMS method.
- Importantly the HE stain can be unreliable for the diagnosis of amyloidosis because it may exhibit similar characteristics to the eosinophilic material seen in glomerulosclerosis.
- Minimal amyloidosis might only be detectable by electron microscopy.
- Non-amyloidotic, non-congophilic fibrillary deposits, with similar tinctorial qualities on PAS, MT, HE, and JMS stains, have been identified in canine glomeruli; therefore, a CR should always be performed for a definitive diagnosis.
Clinical Features of Canine Amyloidosis
- Initial sign is moderate to marked proteinuria (typically UPC > 2), with or without hypoalbuminemia.
- Azotemia develops with nephron loss due to progression of disease.
- In cases where amyloid deposition is consistently located in the medullary interstitium, often with a diffuse and severe distribution (e.g., Chinese Shar Peis), early and more severe development of azotemia with milder proteinuria and hypoalbuminemia would be expected.
- Dogs with glomerular amyloidosis might present with dyspnea due to pulmonary artery thrombosis.
- Markedly proteinuric dogs <2 years old are unlikely to have amyloidosis (Segev et al 2012).
- Other common clinical findings include non-regenerative anemia, leukocytosis, low anti-thrombin activity (and associated thromboembolism), and hypertension. In dogs with extra-renal involvement, other clinical findings may be present, particularly hepatobiliary abnormalities with liver involvement.
- Dogs with amyloidosis are less likely to retain robust urine concentrating ability than other categories of glomerular disease.
- Breeds at risk include the Chinese Shar Peis, English Bulldogs, Beagles, Walker Hounds, and possibly Collies (Olsson et al 2013 and Vaden 2011
MINIMAL AMYLOIDOSIS: Glomeruli might appear histologically normal as very small deposits can be overlooked. Ultrastructural evaluation is required for the diagnosis in these cases.
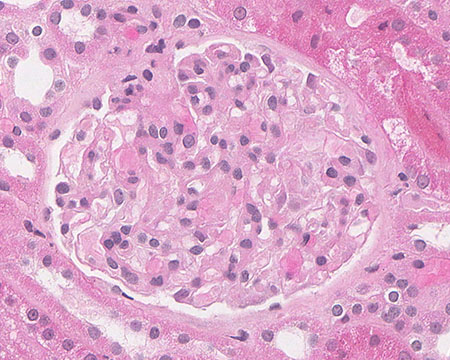
FIG.1A (HE): The glomerulus has a relatively normal histologic appearance.
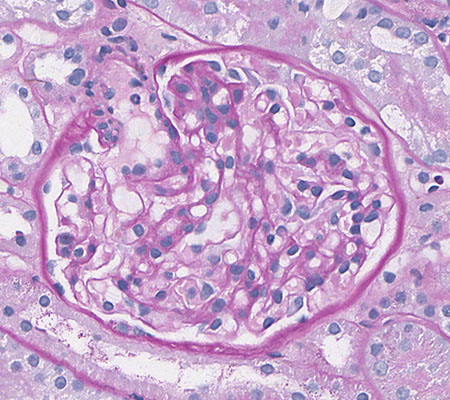
FIG.1B (PAS): The glomerulus has a relatively normal histologic appearance.
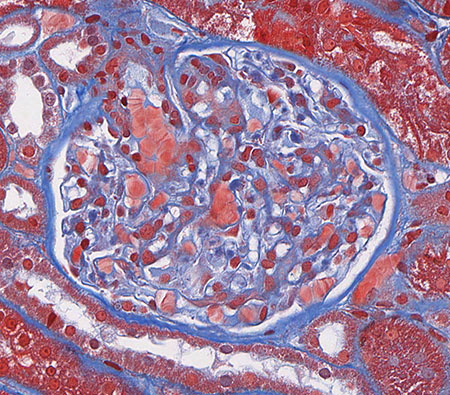
FIG.1C (MT): The glomerulus has a relatively normal histologic appearance.
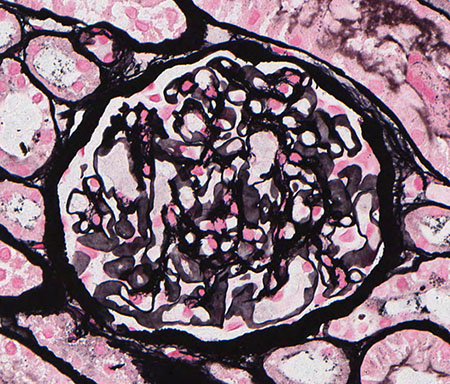
FIG.1D (JMS): The glomerulus has a relatively normal histologic appearance.
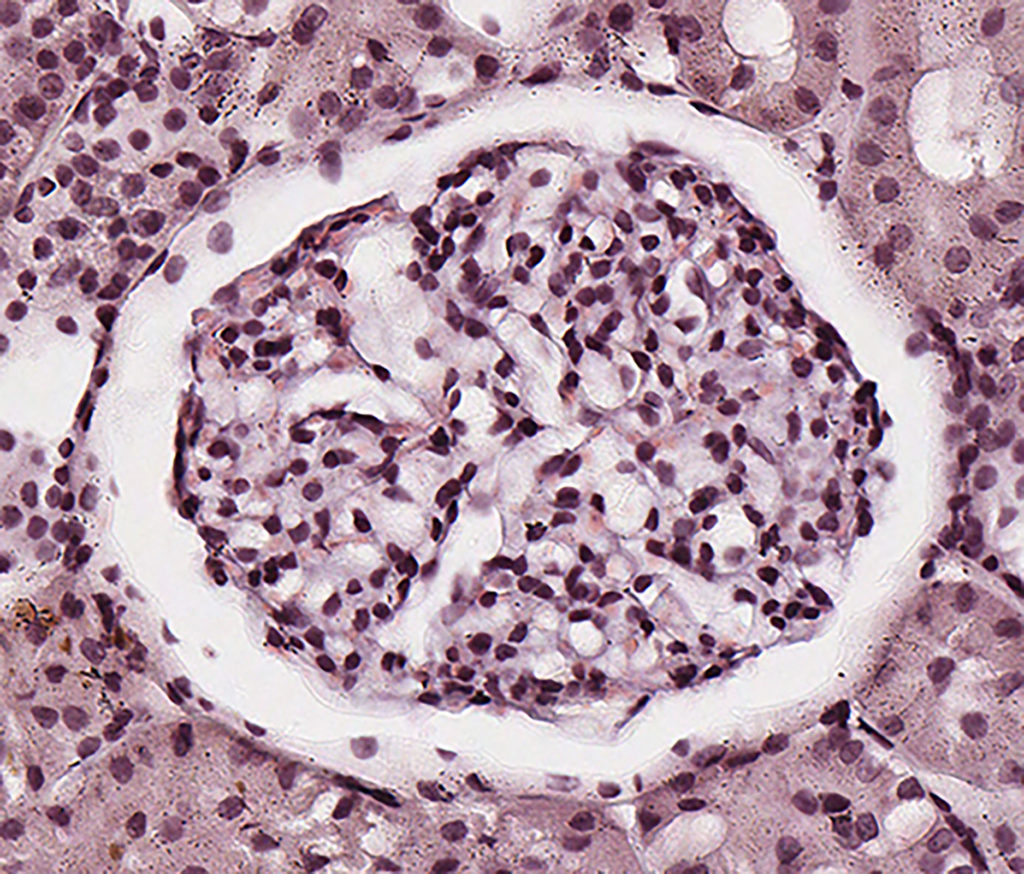
FIG.1E (CR): Glomerulus has a histologically normal appearance; amyloid is not evident. Please note because the sample was cut at 8µm thickness, the glomerulus appears hypercellular.
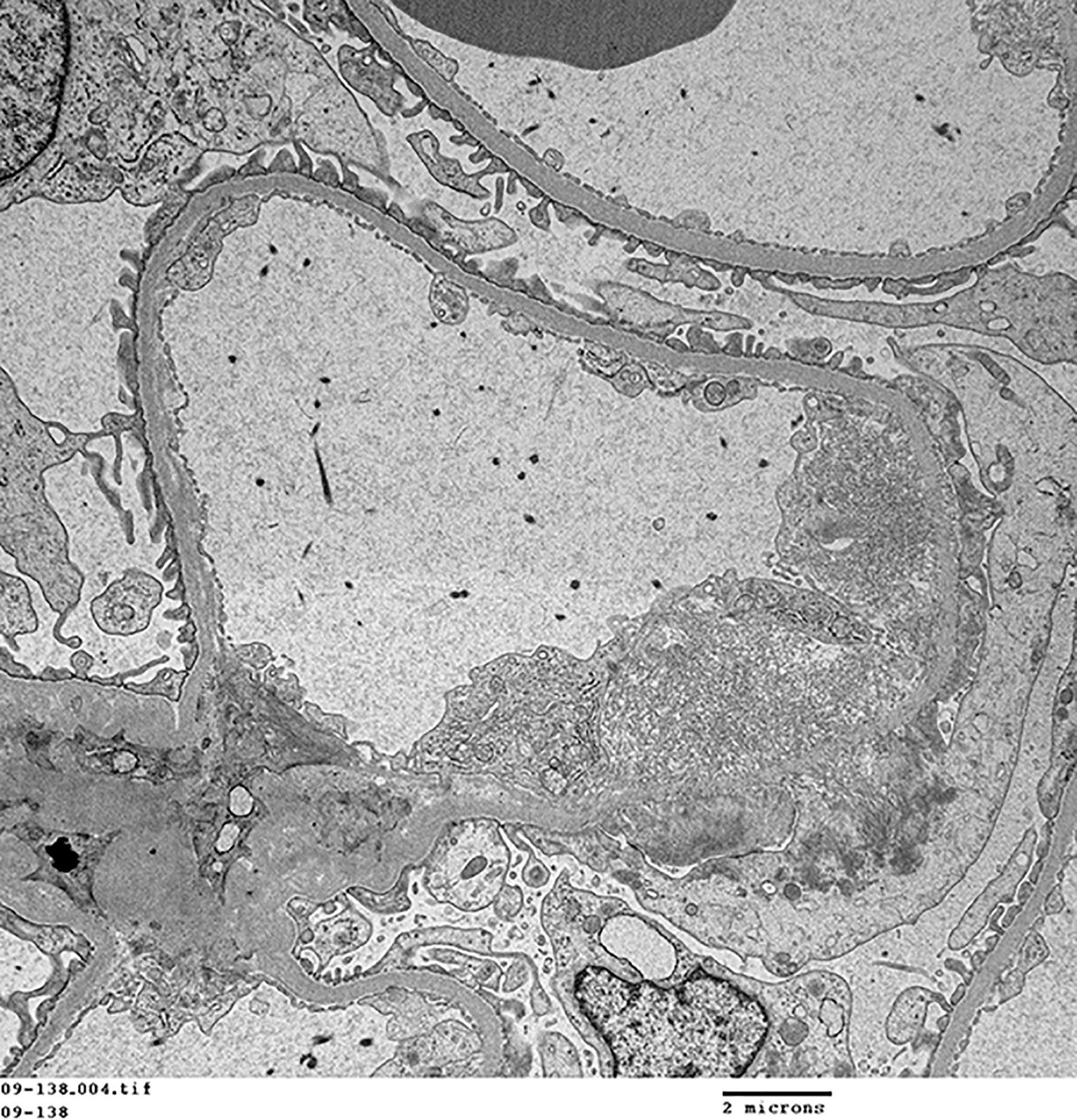
FIG.1F (TEM): There is segmental expansion of the capillary wall beneath the endothelium, with extension across the GBM, by non-branching, non-periodic, haphazardly arranged 9 to 11 nm diameter fibrils. These fibrils are only identified in small segments of the glomeruli. Podocyte foot processes over areas of fibril deposition are effaced.
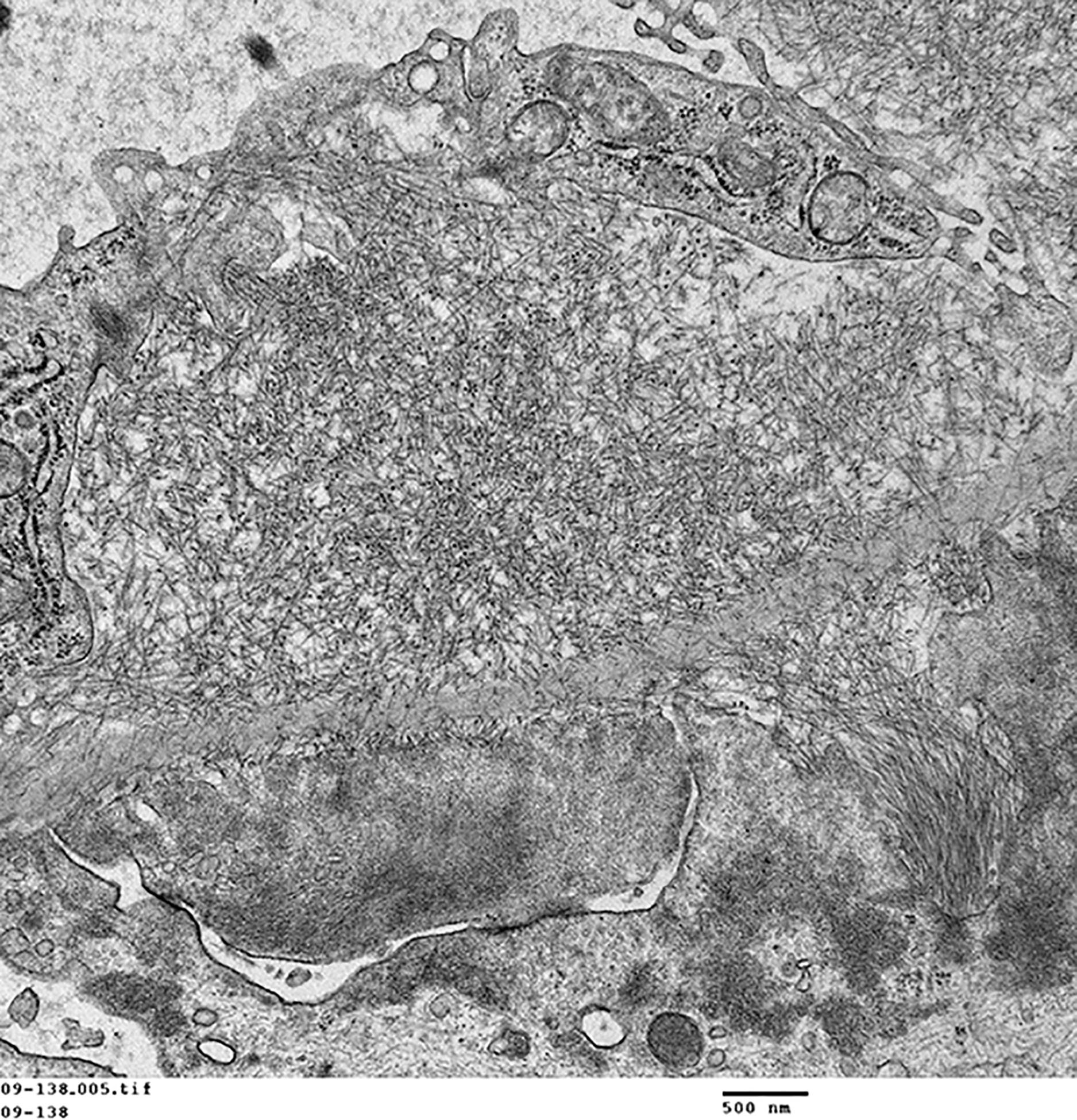
FIG.1G (TEM): Higher magnification of the amyloid fibrils, which cross the GBM and make a small partially organized projection (so-called spicule) towards the urinary space.
FIG.1H (TEM): Colorized version of above TEM image. (Yellow: endothelial cell, Blue: amyloid fibrils, Green: GBM, Pink: podocyte).
MILD AMYLOIDOSIS: Glomeruli have pale scattered deposits expanding most the mesangium and occasionally the capillary loops with rare occlusion of capillary lumina.
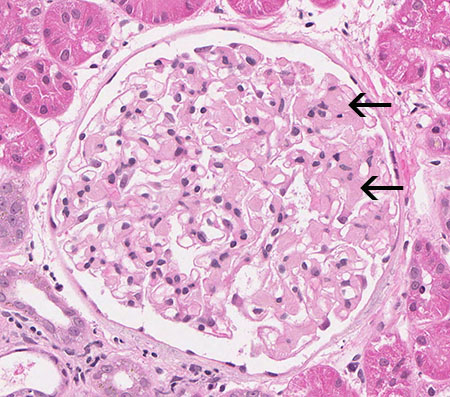
FIG.2A (HE): The mesangium is mildly and multifocally expanded by eosinophilic amorphous material (arrows). The eosinophilia of amyloid is similar to that of glomerulosclerosis; thus HE stain can be unreliable for the diagnosis in mild cases.
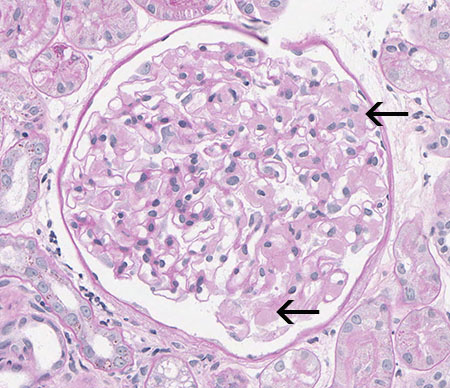
FIG.2B (PAS): Amyloid stains pale pink and waxy in the mesangium and capillary loops (arrows). There is no hypercellularity of the glomerular tuft.
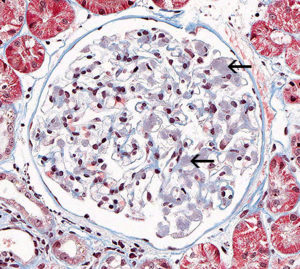
FIG.2C (MT): Amyloid deposits stain mottled blue to orange (arrows).
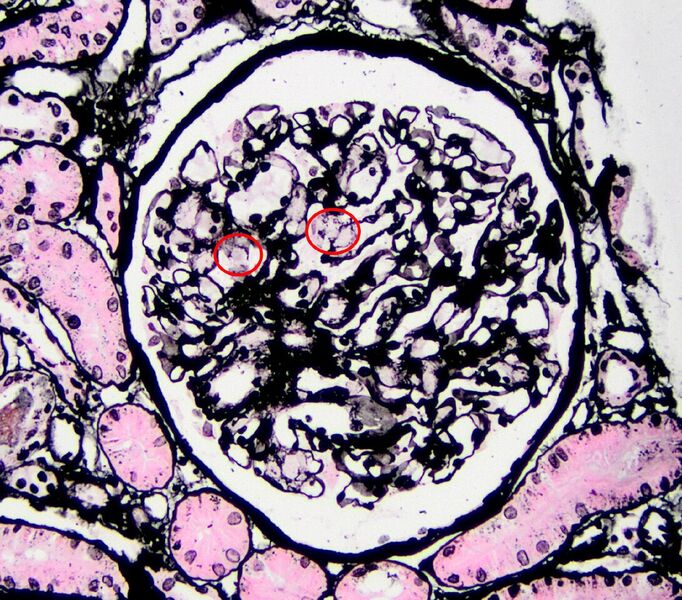
FIG.2D (JMS): Amyloid does not take up silver with the JMS method, and in this case it is a very small amount (circled).
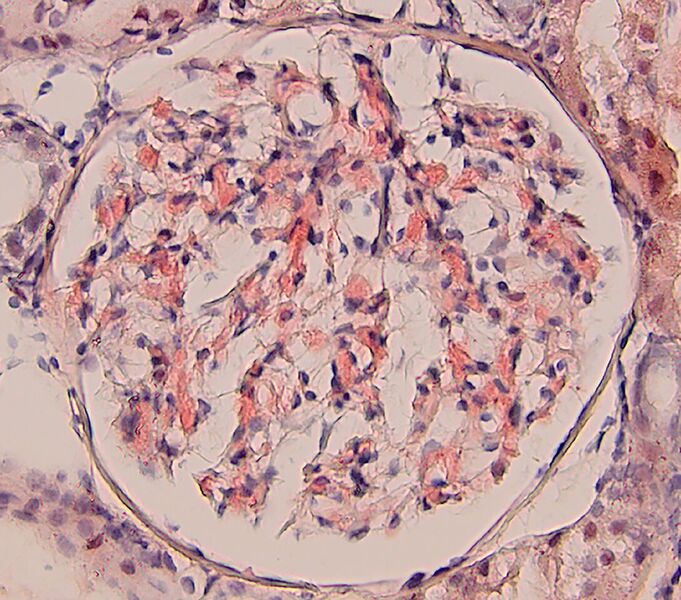
FIG.2E (CR): Amyloid is congophilic and it stains orange to peach. CR positive staining is essential to confirm the diagnosis.
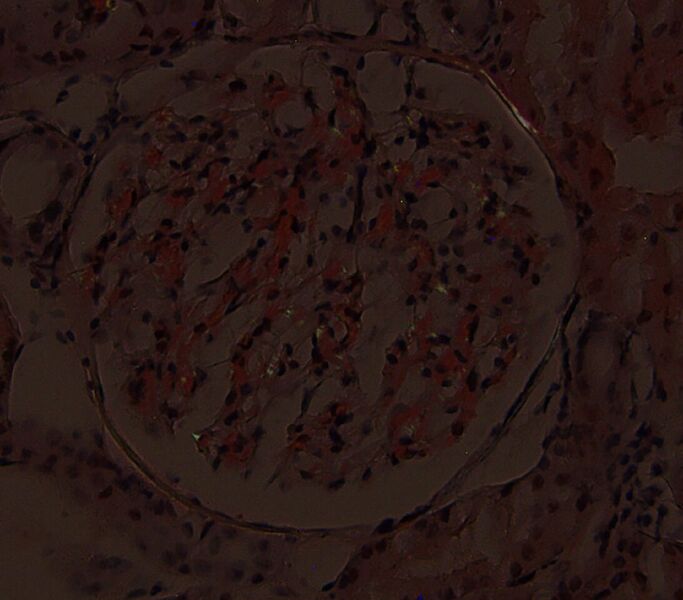
FIG.2F (CR viewed with polarized light): There are small foci of apple green birefringence. Of note, different areas will demonstrate birefringence at various planes of focus on the Z axis.
MODERATE AMYLOIDOSIS: There is diffuse global glomerular deposition of amyloid that widely expands the mesangium and capillary walls with compression and/or effacement of capillary lumina.
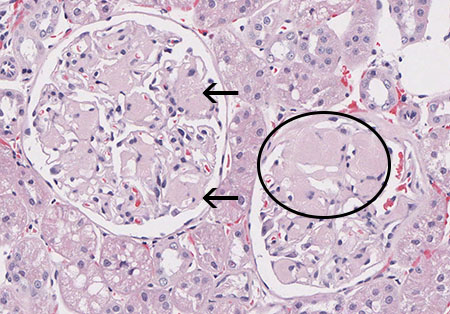
FIG.3A (HE): Mesangial zones are expanded by eosinophilic amorphous extracellular material (arrows) in one glomerulus. The other glomerulus has effacement of peripheral capillary loops by the amyloid (circled) as well as a synechia.
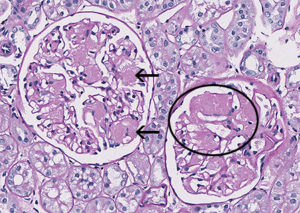
FIG.3B (PAS): Amyloid is pink and waxy (arrows). In the other glomerulus, there is effacement of peripheral capillary loops by the amyloid (circled).
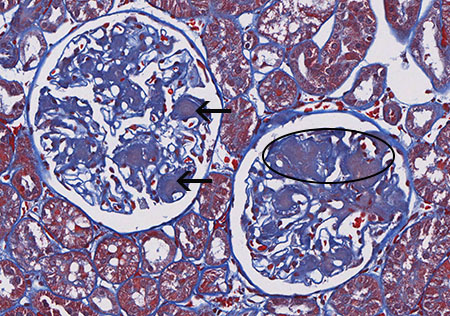
FIG. 3C (MT): Amyloid stains mottled peach to blue (arrows). In the other glomerulus, there is effacement of peripheral capillary loops by amyloid (circled).
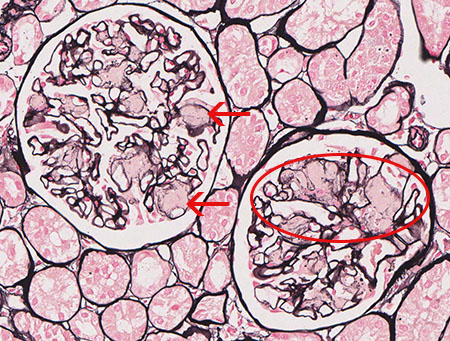
FIG.3D (JMS): Amyloid does not take up silver with the JMS method (arrows). In the other glomerulus, there is effacement of peripheral capillary loops by the amyloid (circled).
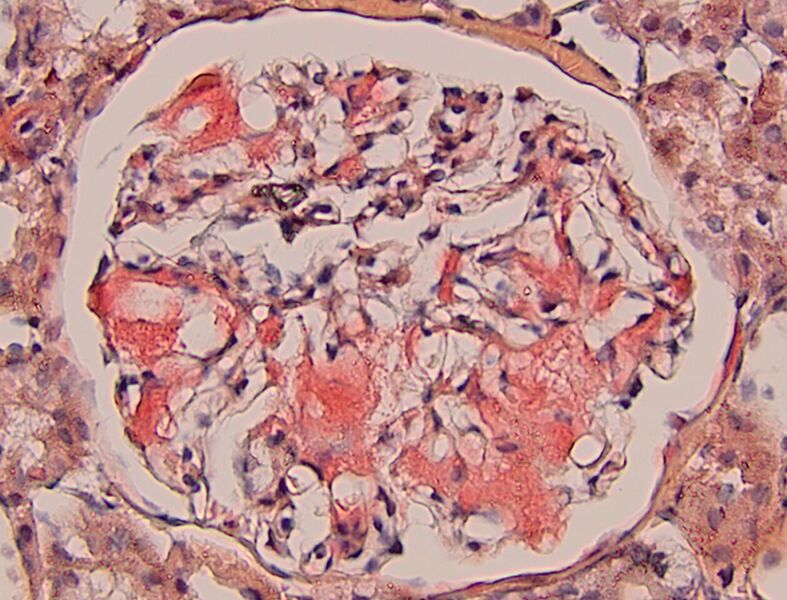
FIG.3E (CR): Amyloid is congophilic thus it stains orange to red with the specific stain. CR positive staining is essential to confirm the diagnosis.
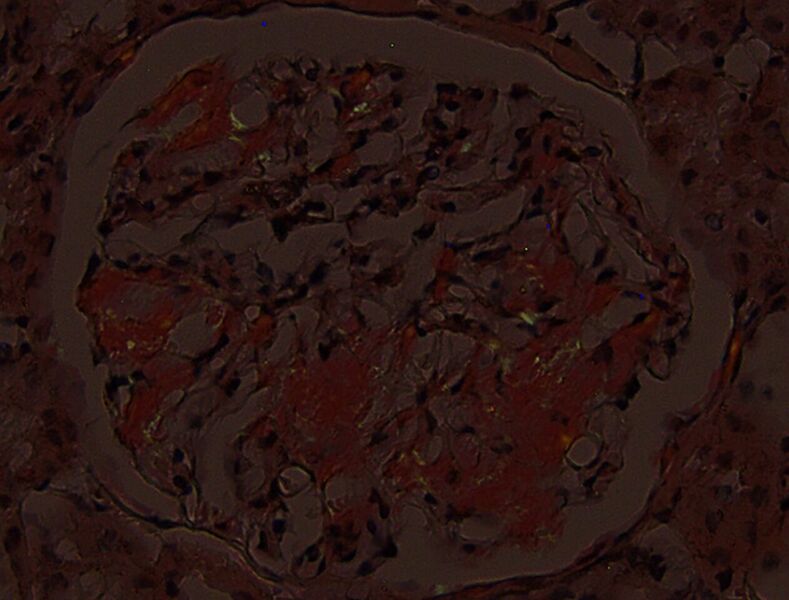
FIG.3F (CR viewed with polarized light): There are small foci of apple green birefringence. Of note, different areas will demonstrate birefringence at various planes of focus on the Z-axis.
SEVERE AMYLOID: Global effacement of most glomeruli by amyloid and there is associated hypocellularity.
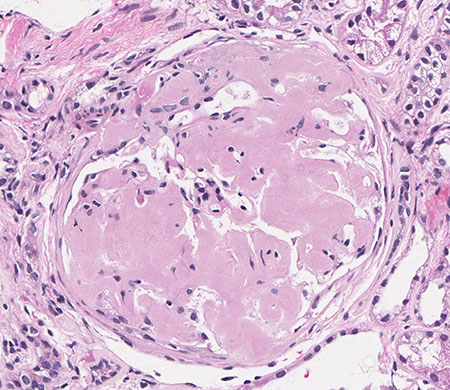
FIG.4A (HE): The major portion of the glomerular tuft is effaced by eosinophilic material and capillary lumena are difficult to identify. Note the surrounding interstitial fibrosis and tubular atrophy.
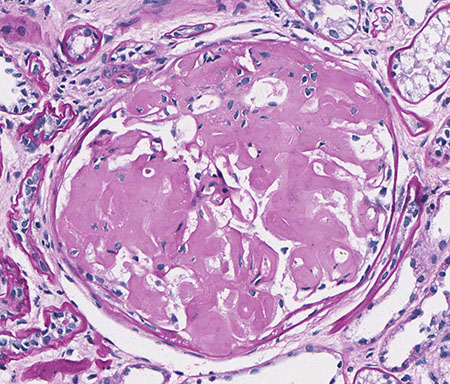
FIG.4B (PAS): Most glomeruli are effaced by waxy, pink material, and capillary lumens are difficult to identify.
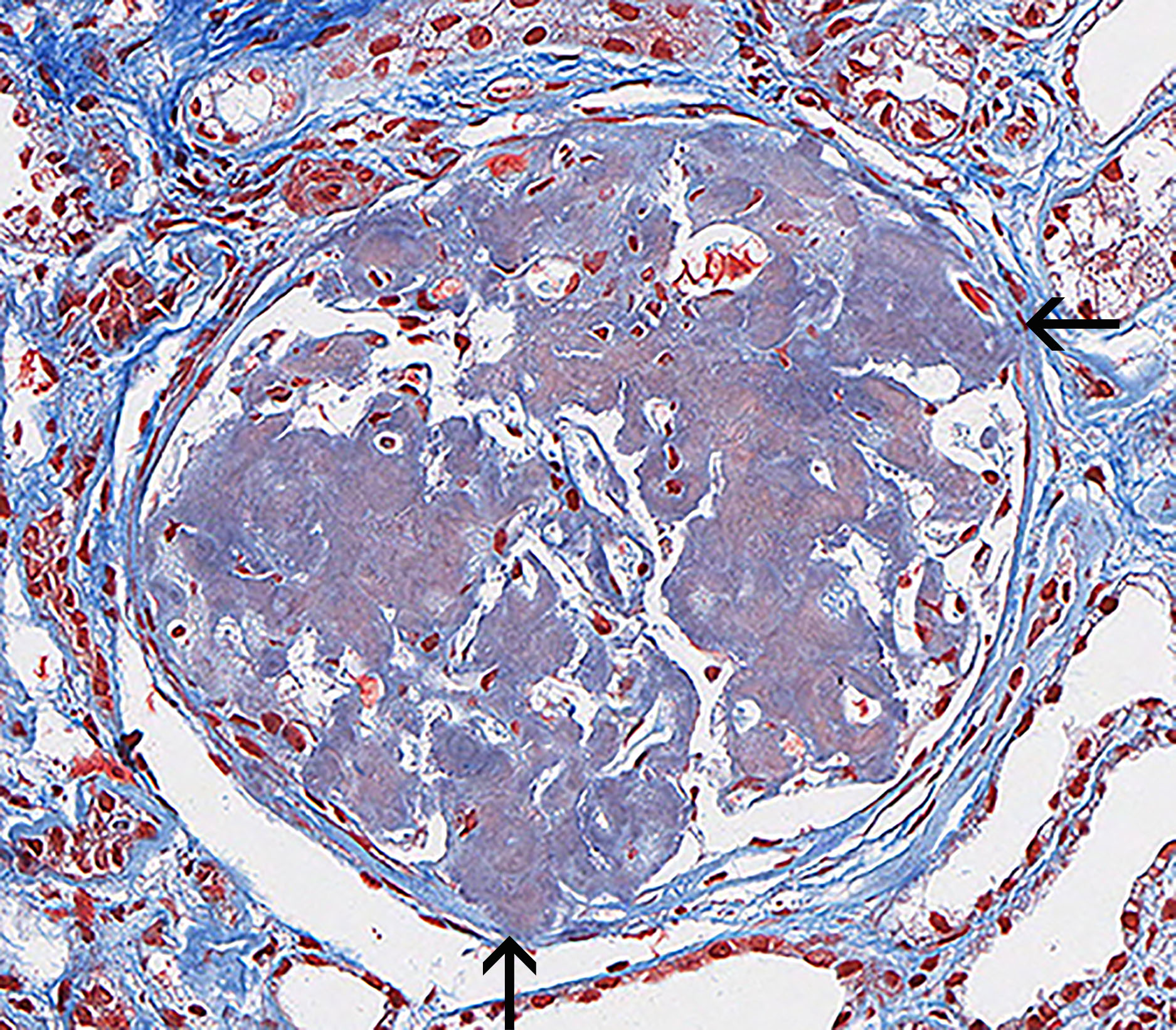
FIG.4C (MT): Amyloid deposits stain mottled blue to orange. Most glomeruli are effaced and capillary lumens are difficult to identify. Synechiae are also present (arrows).
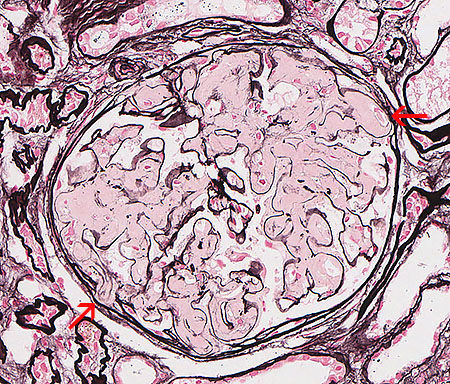
FIG.4D (JMS): Amyloid does not take up silver with the JMS method. Most glomeruli are effaced by eosinophilic material and capillary lumens are difficult to identify. Synechiae are also present (arrows).
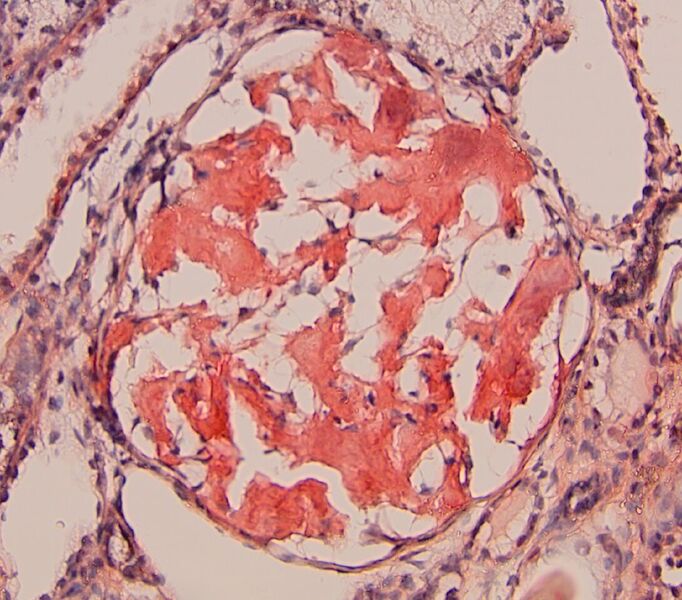
FIG.4E (CR): Amyloid is congophilic thus it stains orange to red with the specific stain. CR positive staining is essential to confirm the diagnosis.
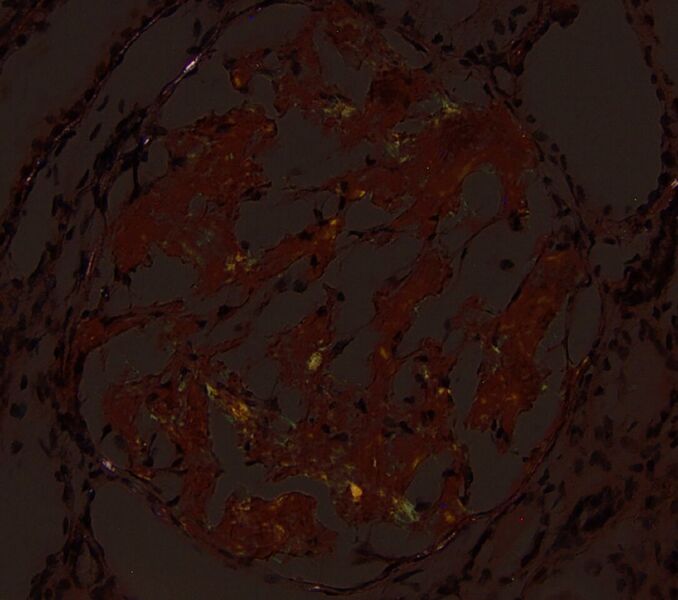
FIG.4F (CR viewed with polarized light): There are small foci of apple green birefringence. Of note, different areas will demonstrate birefringence at various planes of focus on the Z axis.
AMYLOID WITH SPICULES: In some animals with glomerular amyloidosis, the amyloid fibrils are organized into spicules, admixed with GBM material, extending toward the urinary space (see FIG.5C-5F). In humans, this histologic phenotype is called the “Roman helmet” or “coxcomb” appearance. Spicules can only be observed with special stains (MT and JMS) and by TEM.
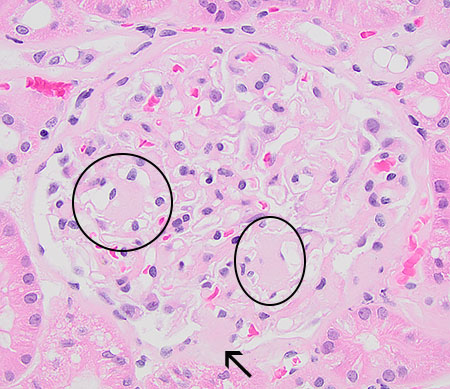
FIG.5A (HE): Mesangium is expanded by eosinophilic material consistent with amyloid (circles) and there is a synechia (arrow).
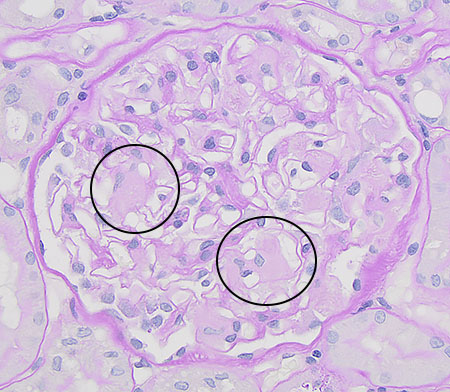
FIG.5B (PAS): Mesangium is expanded by pale pink material consistent with amyloid (circled).
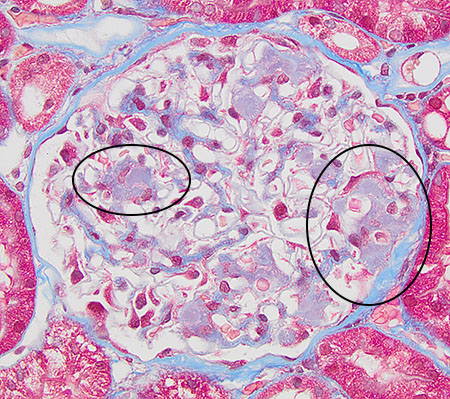
FIG.5C (MT): Mesangium is expanded by pale blue material consistent with amyloid (circled).
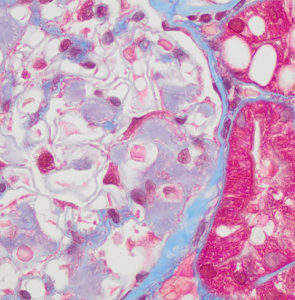
FIG.5D (MT): Higher magnification. There are pale blue spicular projections, representing a combination of amyloid and GBM material, towards the urinary space.
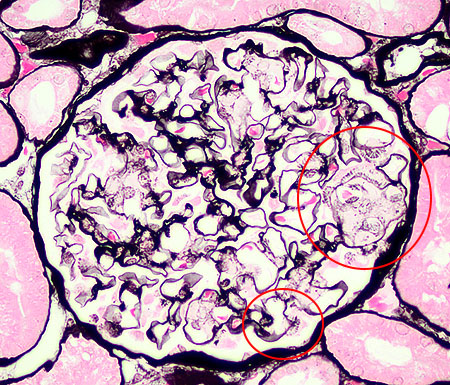
FIG.5E (JMS): There are focal spicular projections of GBM material towards the urinary space, perpendicular to the GBM (circled).
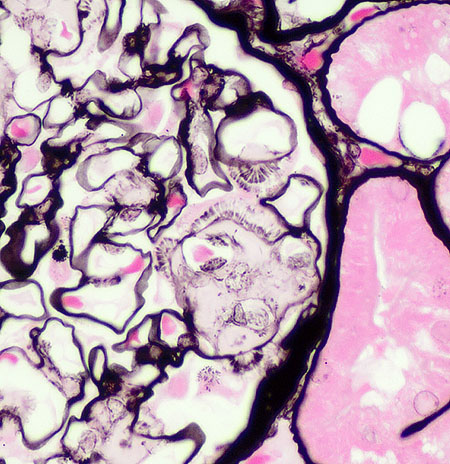
FIG.5F (JMS): Higher magnification of the spicular projections towards the urinary space, the spicules are focal and subepithelial.
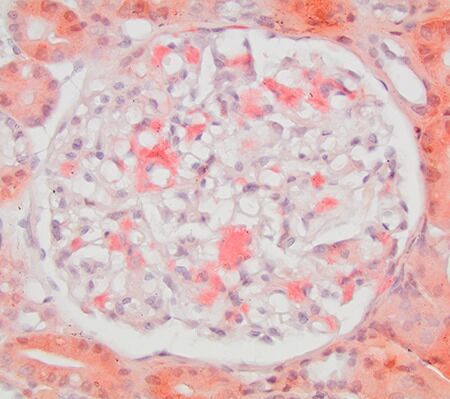
FIG.5G (CR): Congo red material expands the mesangium. Because CR method is performed on a thicker section (8-10 μm instead of 2-3 μm), spicules might be difficult to appreciate with this stain.
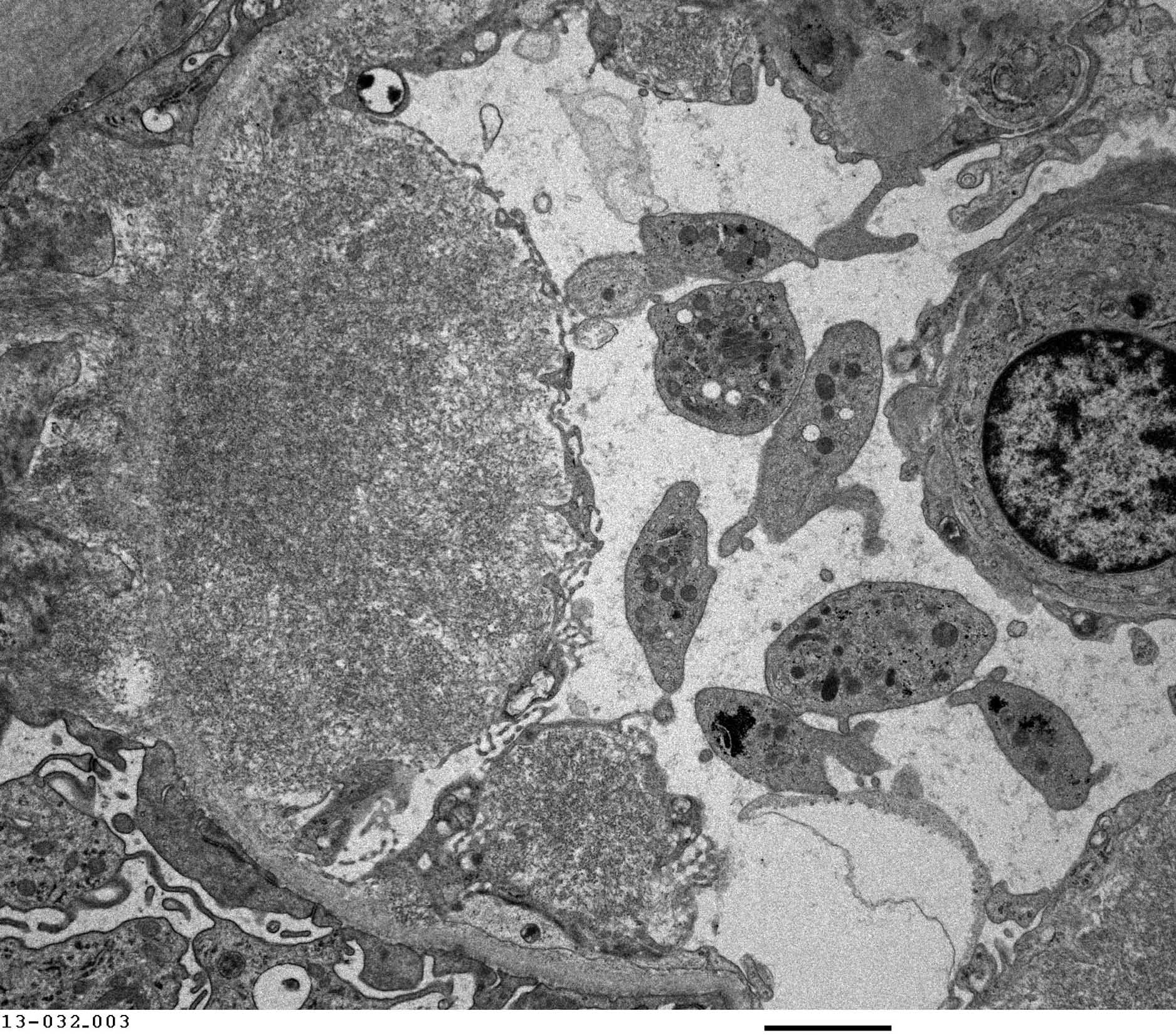
FIG.5H (TEM): Amyloid fibrils are densely packed and organized into small spicules oriented perpendicular to the GBM beneath podocytes. The podocyte is on the left side of this electron micrograph. There are multiple platelets within the capillary lumen.
FIG.5I (TEM): Colorized version of above TEM image. (Yellow: endothelial cell, Blue: amyloid fibrils, Green: GBM, Pink: podocyte). There are multiple platelets within the capillary lumen (uncolored).

FIG.5J (TEM): Higher magnification of the amyloid fibrils demonstrating their haphazard arrangement.
INTERSTITIAL AND VASCULAR AMYLOID: In some animals, amyloid is identified in interstitial regions or vessel walls.
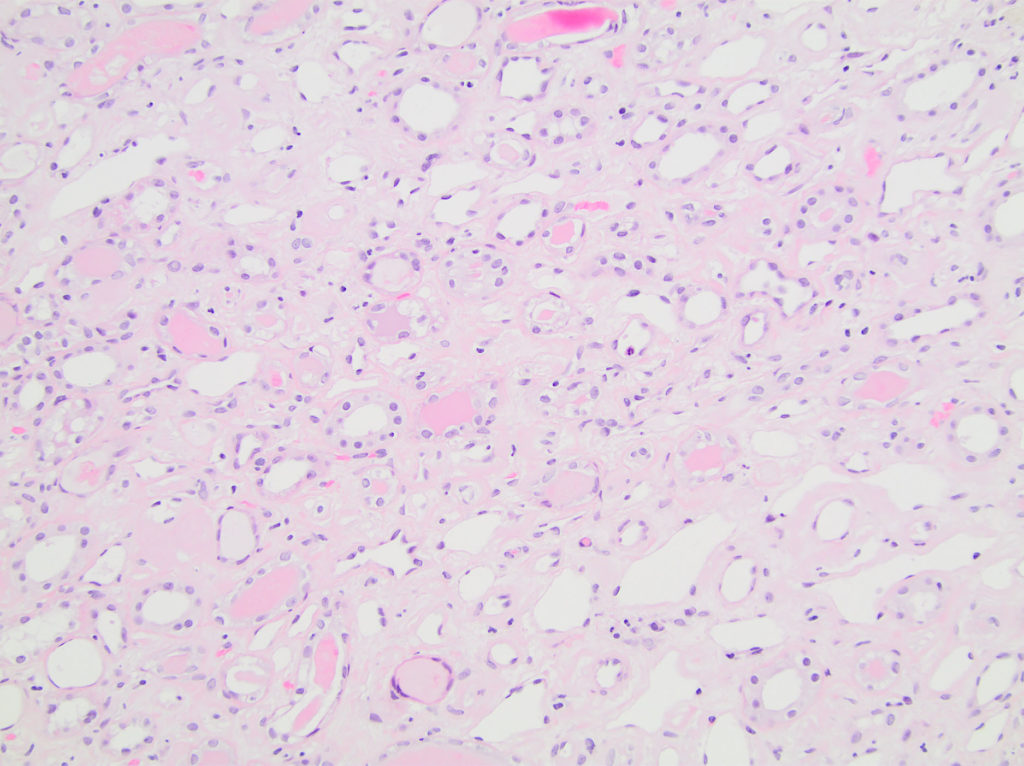
FIG.6A (HE): Medullary interstitium is mildly to moderately expanded by eosinophilic material; many tubules contain protein casts.
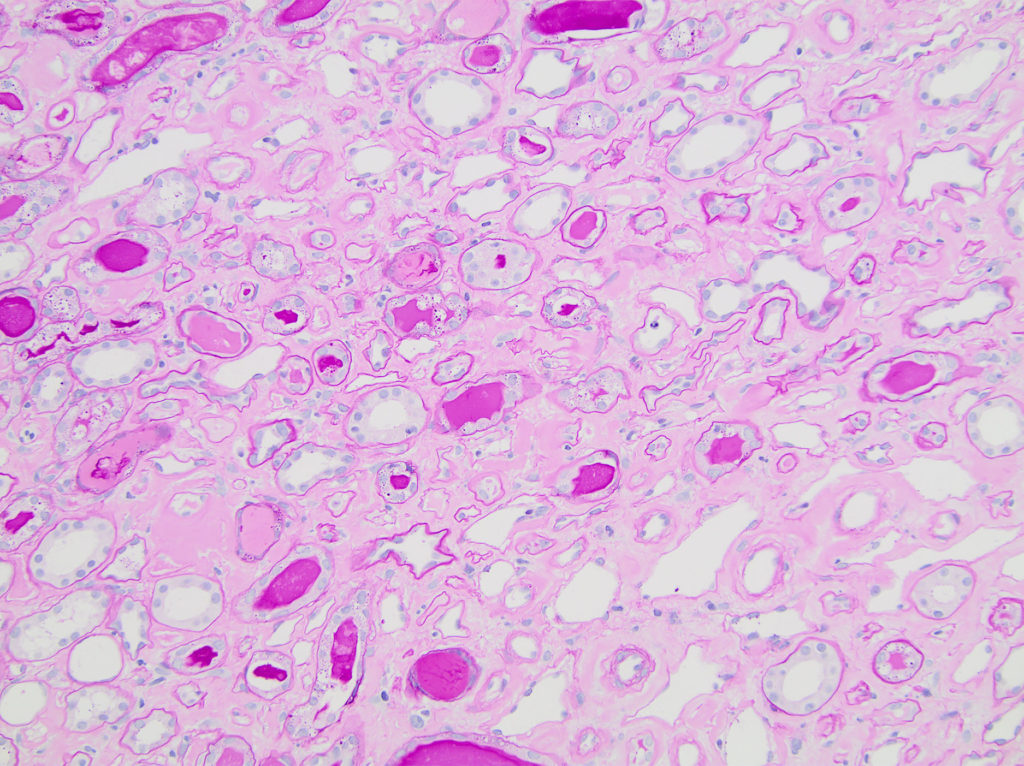
FIG.6B (PAS): Medullary interstitium is mildly to moderately expanded by pale pink material; many tubules are atrophic characterized by wrinkled thickened tubular basement membranes. The intraluminal protein casts are PAS positive, varying from pink to magenta.
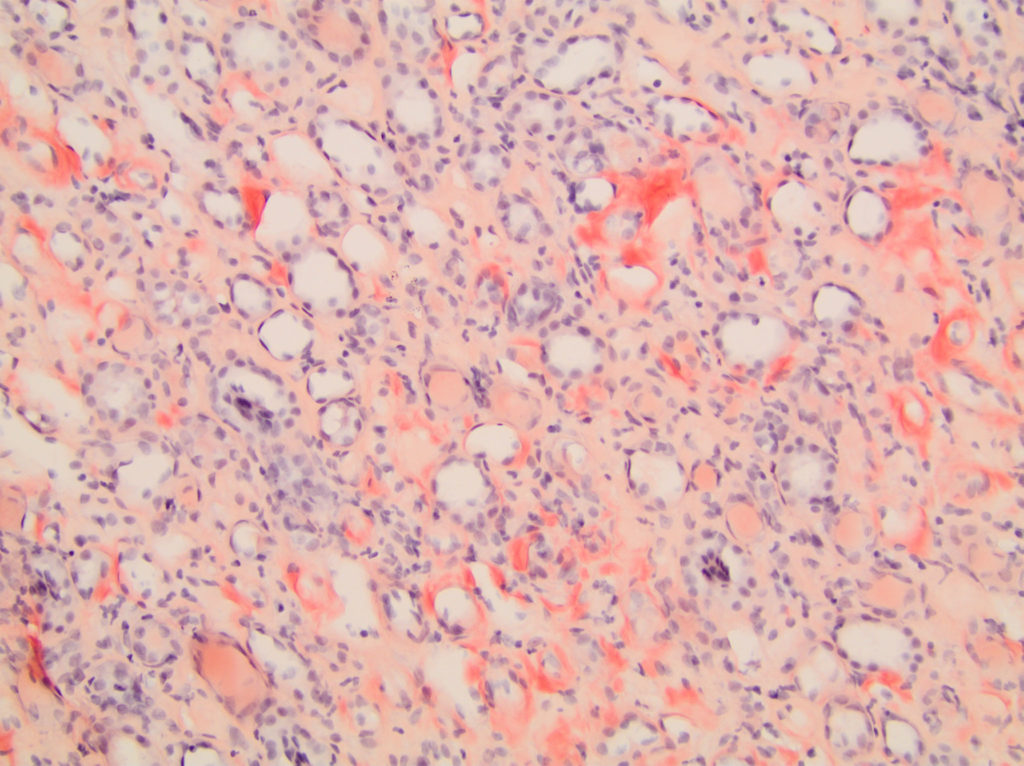
FIG.6C (CR): Medullary interstitium is mildly to moderately expanded by congophilic, peach material.
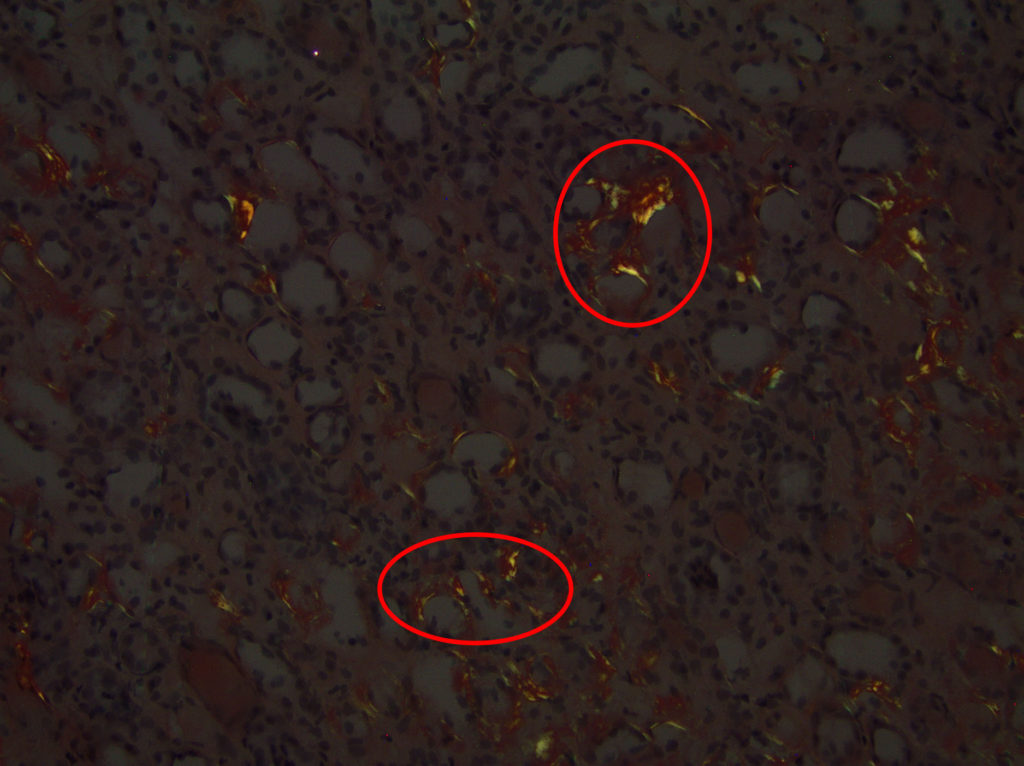
FIG.6D (CR viewed with polarized light): There is apple green birefringent material in the interstitium.
VASCULAR AMYLOIDOSIS: Arterial walls are expanded by congophilic material.
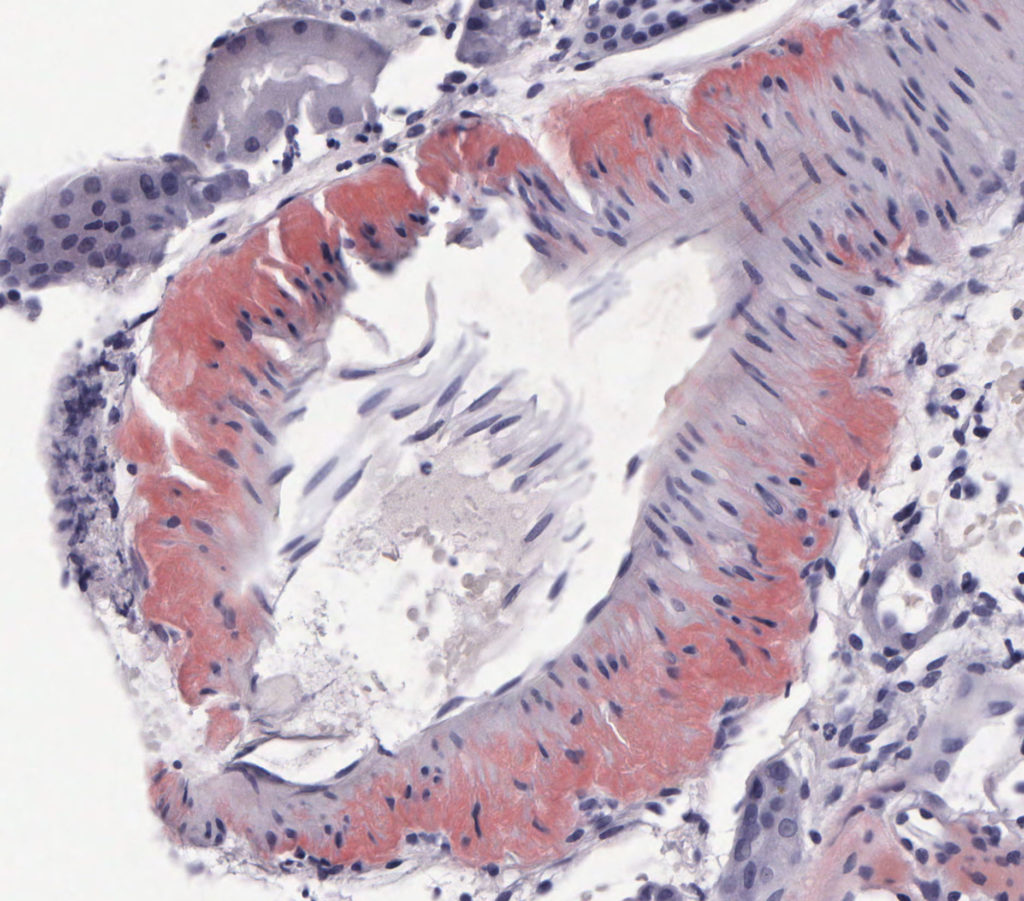
FIG.6E (CR): The wall of an arcuate caliber artery is expanded by congophilic material.
Special Features of Feline Amyloidosis
- Cats with AA amyloid tend to have interstitial amyloidosis, sometimes in the absence of glomerular amyloidosis
- Feline breeds at risk include Siamese, Oriental and Abyssinian breeds (van der Linde-Sipman et al 1997 and Niewold et al 1999).
FIG.7A (HE): There is marked expansion of the mesangium and capillary walls by eosinophilic material. The circled hypercellularity is predominantly mesangial.
FIG.7B (PAS): The material is pale pink on PAS and is outlined by the darker fuschia staining of the GBM. There are multiple synechiae (circled).
FIG.7C (TRI): The material has admixed blue and peach staining (*) and multiple synechiae (circled). There is also associated interstitial and periglomerular fibrosis.
FIG.7D (JMS): The amyloid does not take up silver and therefore amyloidotic regions of the mesangium do not stain black (*). Synechiae are present as well (red circles).
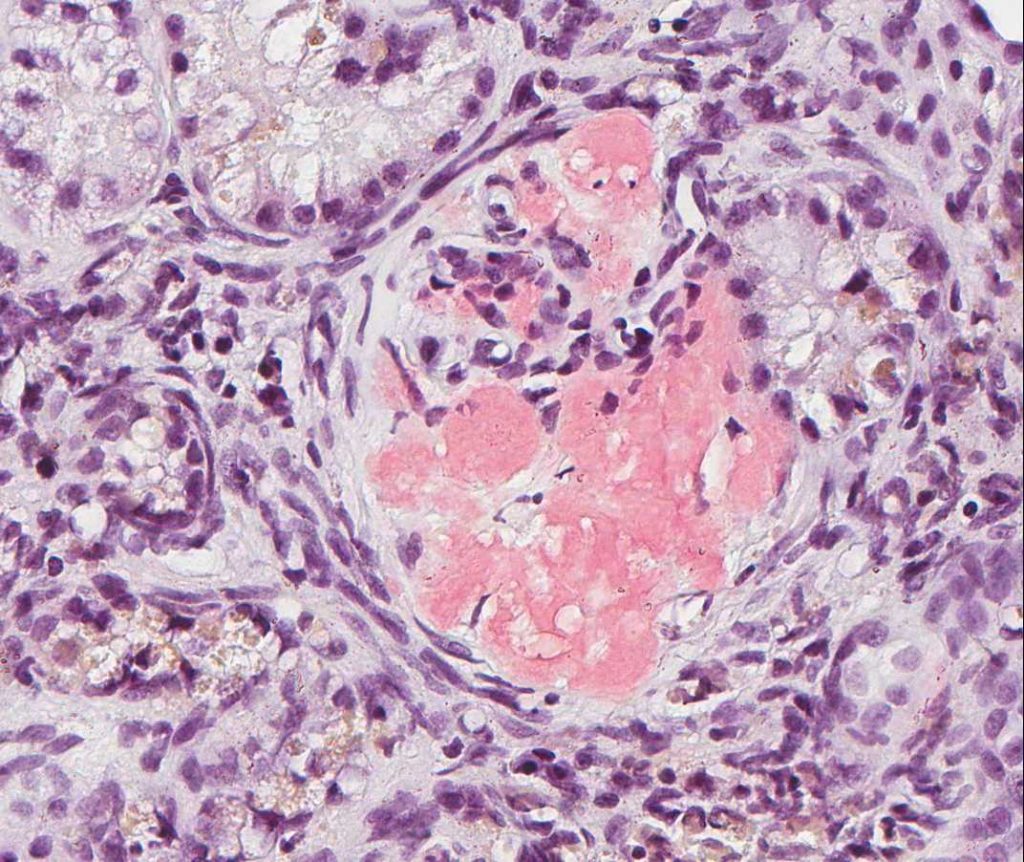
FIG.7E (CR): There is marked expansion of the mesangium and effacement of capillary lumens by Congophilic material.
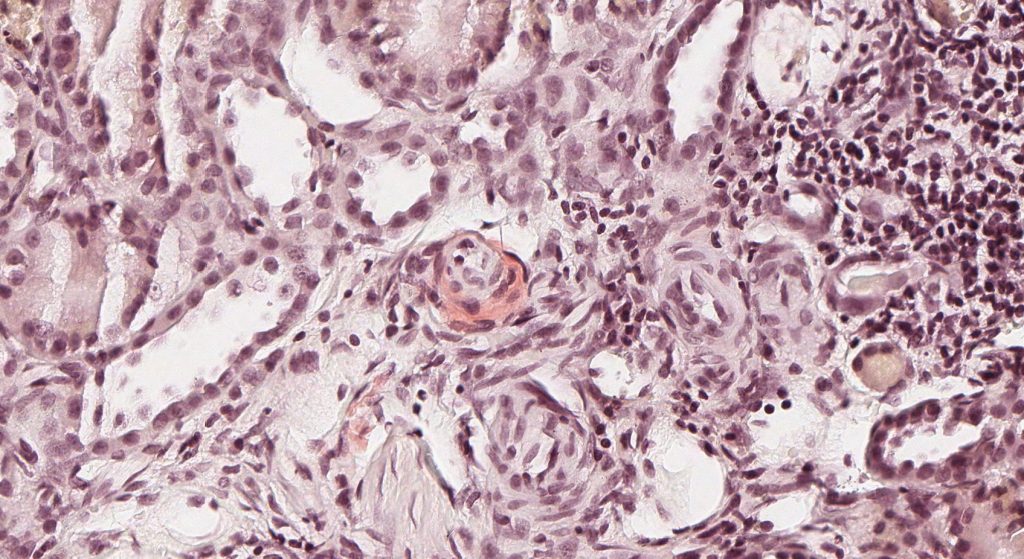
FIG.7F (CR): There is congophilic material encircling the arteriole. Similar small amounts of amyloid was present within the interstitium and around small caliber vessels.
FIGURE 8: ADDITIONAL DIAGNOSTICS FOR AMYLOID: TEM reveals shows are haphazardly arranged fibrils (9-11nm in diameter) in glomeruli. IF demonstrates strong non-specific labeling of glomeruli.
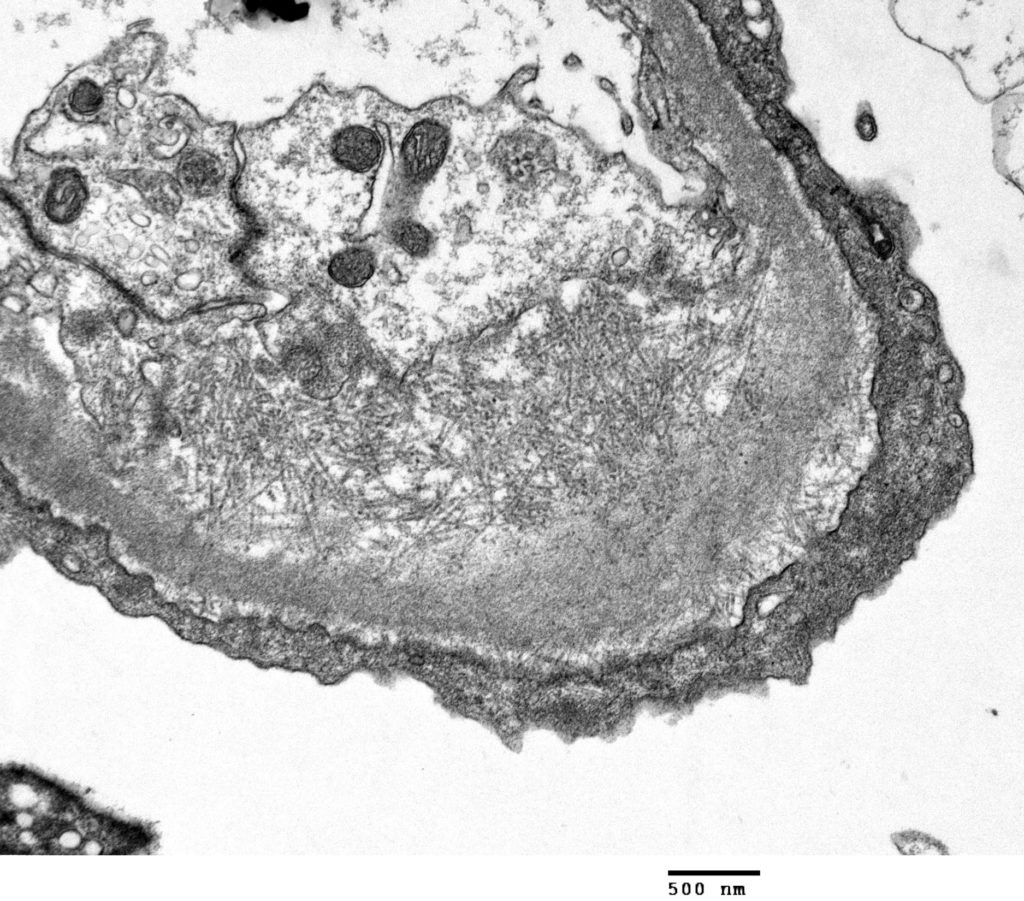
FIG.8A (TEM): There is subendothelial expansion due to the presence of haphazardly arranged fibrils, 9 to 11 nanometers in diameter. Some fibrils cross the GBM. The podocyte foot processes are effaced.
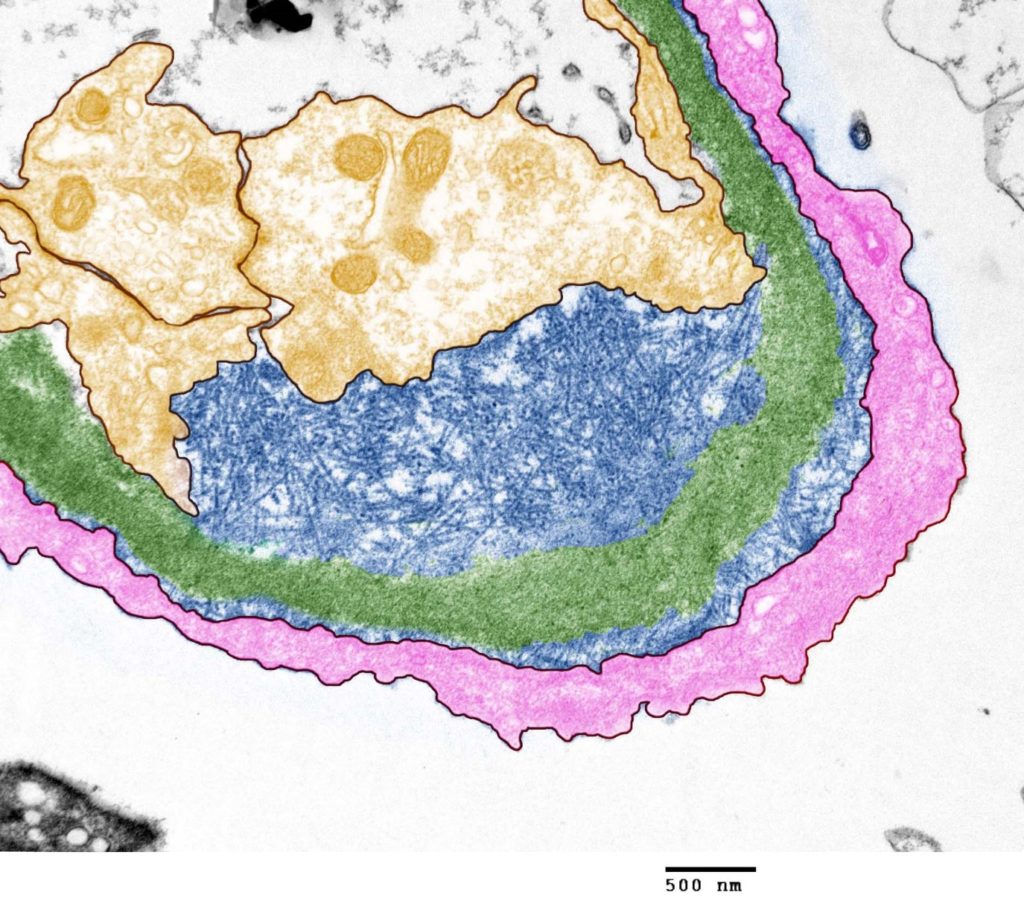
FIG.8B (TEM): Colorized version of previous TEM image (Yellow: endothelial cell, Blue: amyloid fibrils, Green: GBM, Pink: podocyte).
IF OF AMYLOIDOSIS: Strond non-specific labeling of glomeruli.
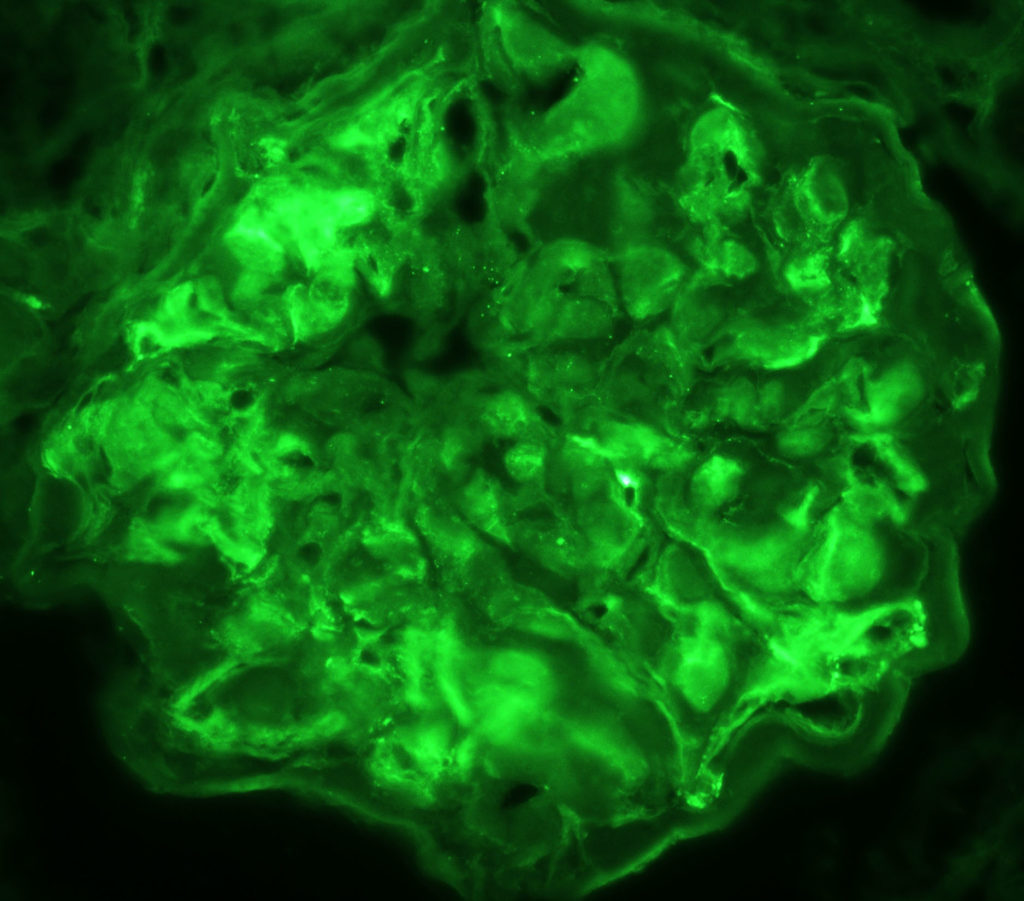
FIG.8C (IF for LLC): Granular labeling is not identified; however, there is moderate to strong splotchy non-specific labeling of the glomerular tuft due to entrapment of normal plasma proteins within the amyloid fibrils.
Differential Diagnoses for Amyloidosis:
| Disease | Defining Histologic Features | Defining Ultrastructural Features |
| Amyloidosis |
|
Haphazardly arranged, non-branching fibrils, 9 to 11nm in diameter |
| Non-amyloidotic fibrils |
|
Haphazardly arranged fibrils > 12 nm and are often > 15nm |
| Glomerulosclerosis |
|
Discrete fibrils are not identified. |
Key Diagnostic Features of Amyloidosis
- Expansion of the mesangium and / or the capillary walls by extracellular material to a similar degree in all glomeruli.
- The material stains pink with HE, pale pink with PAS, orange or blue with MT, does not take up silver with the JMS, is orange / peach with the CR method, and exhibits apple green birefringence when the CR is viewed with polarized light.
- Ultrastructurally, fibrils are 9 to 11nm in diameter and do not branch. They are usually haphazardly arranged but can form spicular aggregates along the capillary walls with corresponding remodeling of the GBM material.
- IF can demonstrate bright non-specific labeling.
- Amyloid can also be present in the interstitium and in vasculature. Interstitial deposition more likely to be associated with azotemia.
Amyloidosis References
- Segev, G., Cowgill, L. D., Jessen, S., Berkowitz, A., Mohr, C., Aroch, I. (2012). Renal Amyloidosis in Dogs: A Retrospective Study of 91 Cases with Comparison of the Disease between Shar-Pei and Non-Shar-Pei Dogs. Journal of Veterinary Internal Medicine, 26(2): 259-268.
- Olsson, M., Tintle, L., Kierczak, M., Perloski, M., Tonomura, N., Lundquist, A., Meadows, J. R. S. (2013). Thorough Investigation of a Canine Autoinflammatory Disease (AID) Confirms One Main Risk Locus and Suggests a Modifier Locus for Amyloidosis. PLoS ONE, 8(10): e75242.
- Schneider, S.M., Cianciolo, R.E., Nabity, M.B., Clubb, F.J., Brown, C.A. Lees, G.E. (2013), Prevalence of Immune-Complex Glomerulonephritides in Dogs Biopsied for Suspected Glomerular Disease: 501 Cases (2007–2012). J Vet Intern Med, 27: S67–S75.
- Cianciolo, R.E., Mohr, F.C., Aresu, L., Brown, C.A., James, C., Jansen, J.H., Spangler, W.L., van der Lugt, J.J., Kass, P.H., Brovida, C., Cowgill, L. D., Heiene, R., Polzin, D.J., Syme, H., Vaden, S.L., van Dongen, A., Lees, G. E. (2016), World Small Animal Veterinary Association Renal Pathology Initiative: Classification of Glomerular Disease in Dogs. Vet Pathol, 53(1):113-135.
- Vaden, S. (2011), Glomerular Disease. Topics in Comp Anim Med, 26(3), 128-134.
- DiBartola, S.P., Tarr, M.J., Parker, A.T., Powers, J.D., Pultz, J.A (1989), Clinicopathologic findings in dogs with renal amyloidosis: 59 cases (1976-1986). JAVMA, 195(3): 358-64.
- van der Linde-Sipman, J.S., Niewold, T.A., Tooten, P.C.J., de Neijs-Backer, M., Gruys, E. (1997), Generalized AA-amyloidosis in Siamese and oriental cats. Vet Immunol and Immunopathology, 56(1-2): 1-10.
- Niewold, T.A., van der Linde-Sipman, J.S., Murphy, C., Tooten, P.C.J., Gruys, E. (1999), Familial amyloidosis in cats: Siamese and Abyssinian AA proteins differ in primary sequence and pattern of deposition. Amyloid, 6(3): 205-209.
