Main Body
Juvenile Onset Chronic Kidney Disease (Juvenile Nephropathy)
JUVENILE-ONSET CHRONIC KIDNEY DISEASE
Please note that the diseases and lesions discussed in this chapter might not always result in proteinuria. They are included here to provide a comprehensive overview of canine renal diseases. In our experience, diagnosis of juvenile onset kidney diseases is a common motivation for obtaining a renal biopsy. Some of the diseases listed below have a known pathogenesis (discussed at the beginning of the chapter) whereas others are likely due to disordered nephrogenesis (discussed at the end).
- The terms “Juvenile-onset chronic kidney disease” (JOCKD) and “juvenile nephropathy” (JN) are loosely defined as any non-inflammatory, degenerative, or developmental chronic kidney disease in young animals (approximately 2 years of age or less), although clinical signs might not become apparent until later in life. In many cases, the pathogenesis might not yet be known. They are not necessarily congenital diseases, and JOCKD is often a diagnosis of exclusion. More restrictive definitions apply to diseases observed within families or breeds, and they are termed familial nephropathy and breed nephropathy, respectively. “Hereditary nephropathy” is the term used once the inheritance pattern of a nephropathy has been determined. Our approach in this chapter is to discuss juvenile renal diseases for which the pathogenesis is fairly well-established and/or definitive diagnostic criteria exist. Then we will broach the topic of abnormal kidney development in utero or during the neonatal period.
HEREDITARY GLOMERULAR DISEASES
- These are a group of progressive glomerular diseases. The genetic causes of some, but certainly not all, have been identified.
- Light microscopic features are not pathognomonic, but they are indicative of a primary glomerular disease with secondary involvement of the other renal compartments.
- Glomeruli can be hypercellular, with segmental to global mesangial expansion.
- Cystic glomerular atrophy is often prominent.
- Tubulointerstitial changes are varied and can include: interstitial inflammation, fibrosis, tubular atrophy and dilation with intraluminal collection of proteinaceous material.
- These secondary lesions (glomerulosclerosis, glomerulocystic atrophy and tubulointerstitial injury) can obscure the primary cause. However, they are important to mention because the marked degree of injury in young dogs might be the initial clue that raises the index of suspicion for a hereditary glomerular injury.
COLLAGENOFIBROTIC GLOMERULOPATHY (Collagen Type III Glomerulopathy)
- Rare glomerulopathy characterized by expansion of mesangium and capillary wall by massive accumulations of type III collagen fibrils, lesser amounts of type V collagen, and fibronectin.
- It affects all glomeruli and, similar to amyloidosis, it can be mild, moderate, or severe according to the dimension and confluence of the deposits and the effacement of tuft architecture.
- With routine histochemical staining, it is somewhat similar to amyloidosis but deposits are not congophilic, and they lack the nodular character typical of amyloid. Because the mesangial expansion is mostly collagen, the material will stain dark grey to black with JMS, whereas amyloid will not take up silver. Electron microscopy reveals large cross-banded fibrils within the widened GBM and mesangium. Positive immunostaining for type III collagen further confirms this diagnosis.
- The typical clinical picture is a young dog (either sex) with marked proteinuria and eventually azotemia. It is a progressive disease, and a cure is not available.
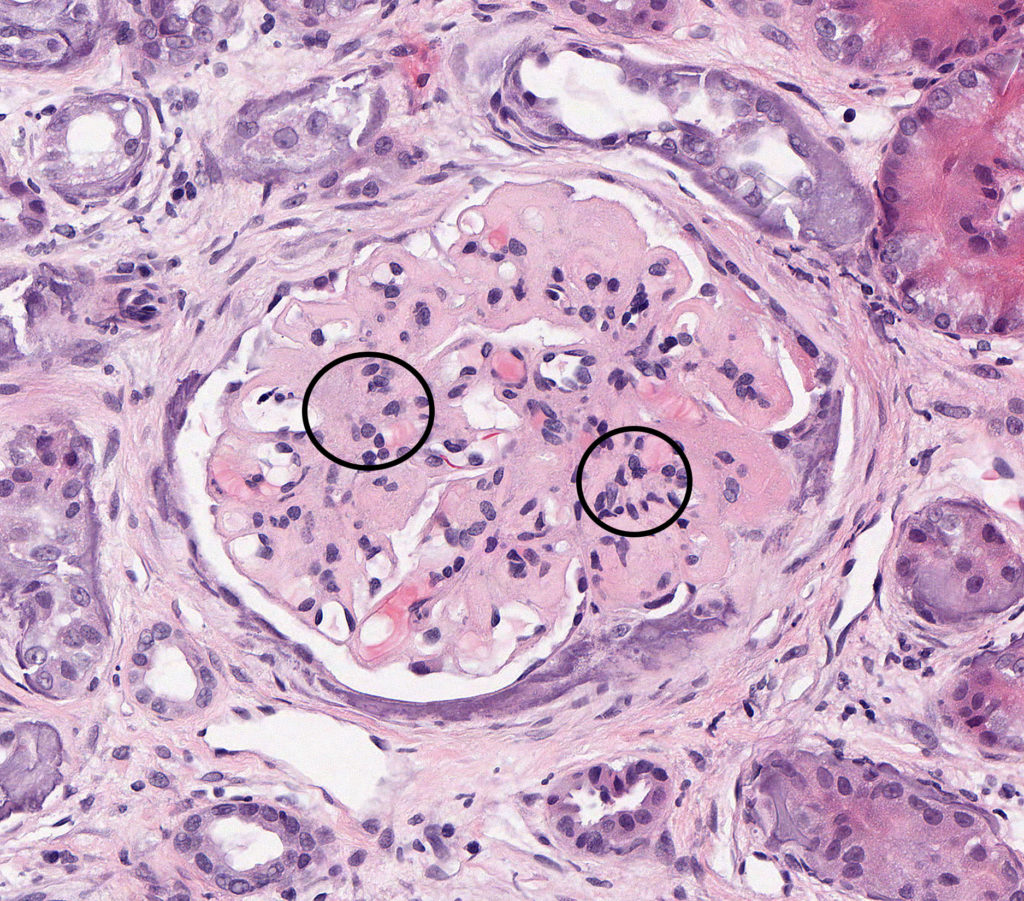
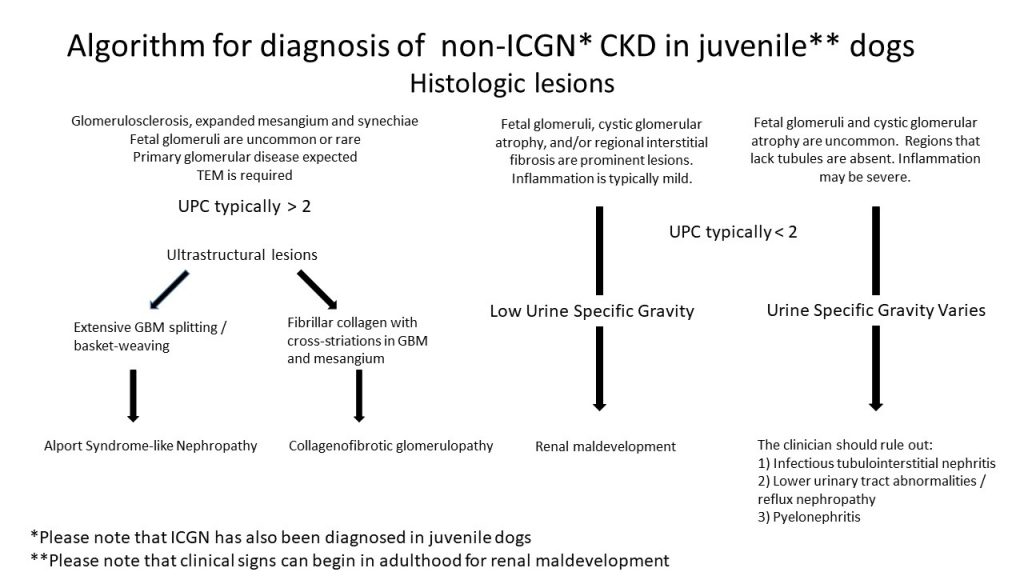
FIG.1A (HE): There is global expansion of the mesangium and capillary loops by eosinophilic material with multifocal collapse of capillary lumina. On HE the material closely resembles amyloid, and other histochemical staining is necessary to differentiate the two diseases. Segmental to diffuse mesangial hypercellularity is observed (circles). Mineralization of the Bowman’s capsule and small synechiae are also present in this image.
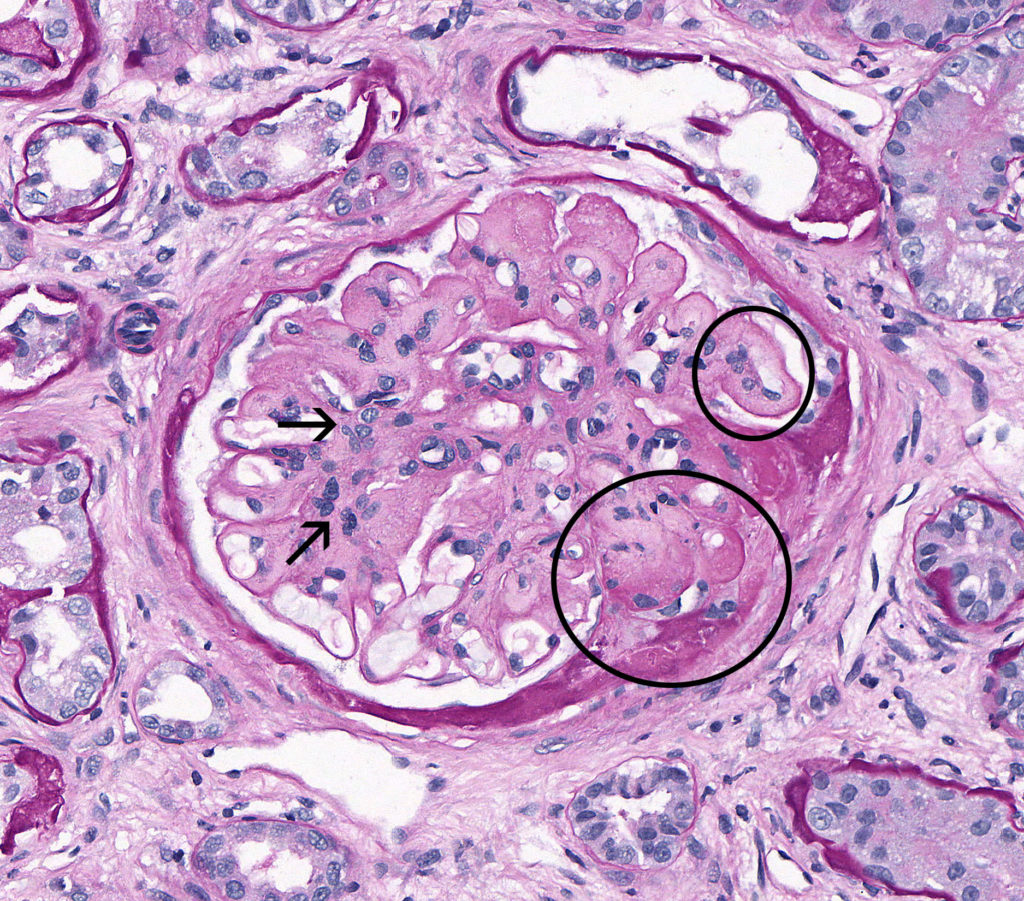
FIG.1B (PAS): The deposited material is weakly positive on PAS. There is expansion of the mesangium and capillary loops with narrowing and partial collapse of capillary lumina (circled). Segmental to diffuse mesangial hypercellularity is observed (arrows).
FIG.1C (MT): Non-amyloidotic fibrillary deposits stain deep blue. Segmental to diffuse mesangial hypercellularity is observed (arrow).
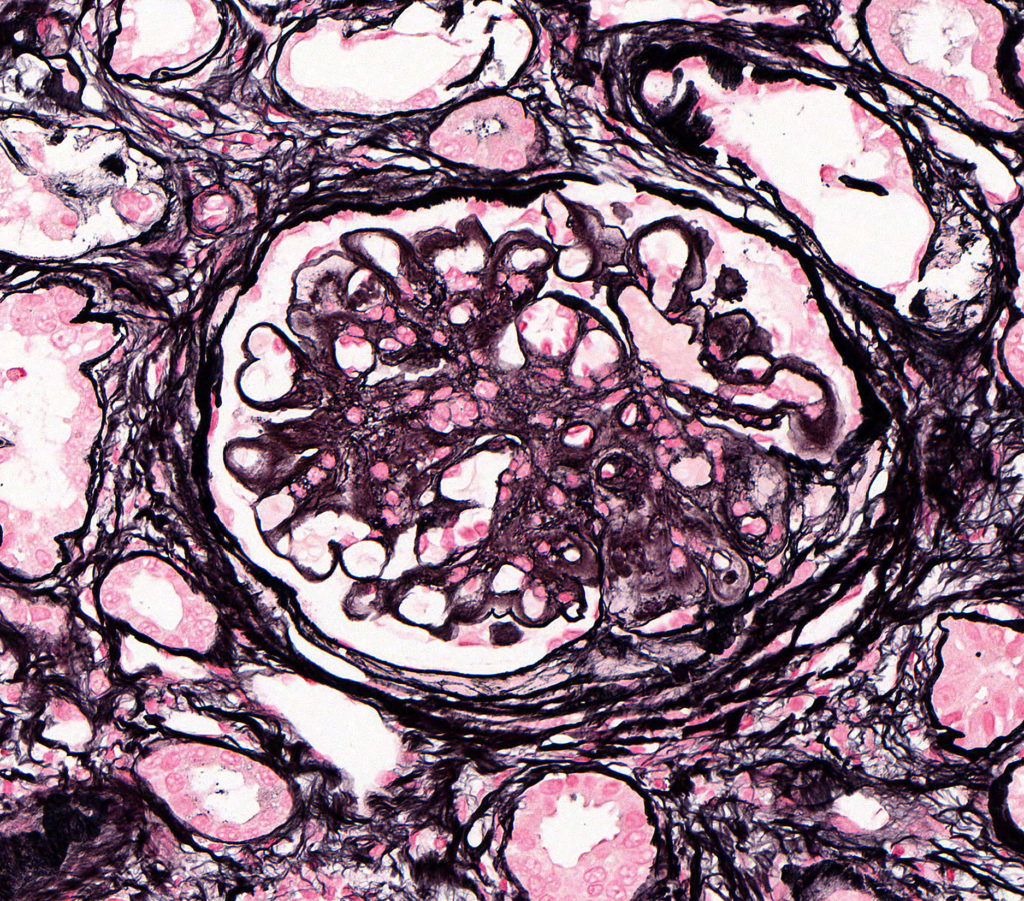
FIG.1D (JMS): Deposits take up silver with the JMS method.
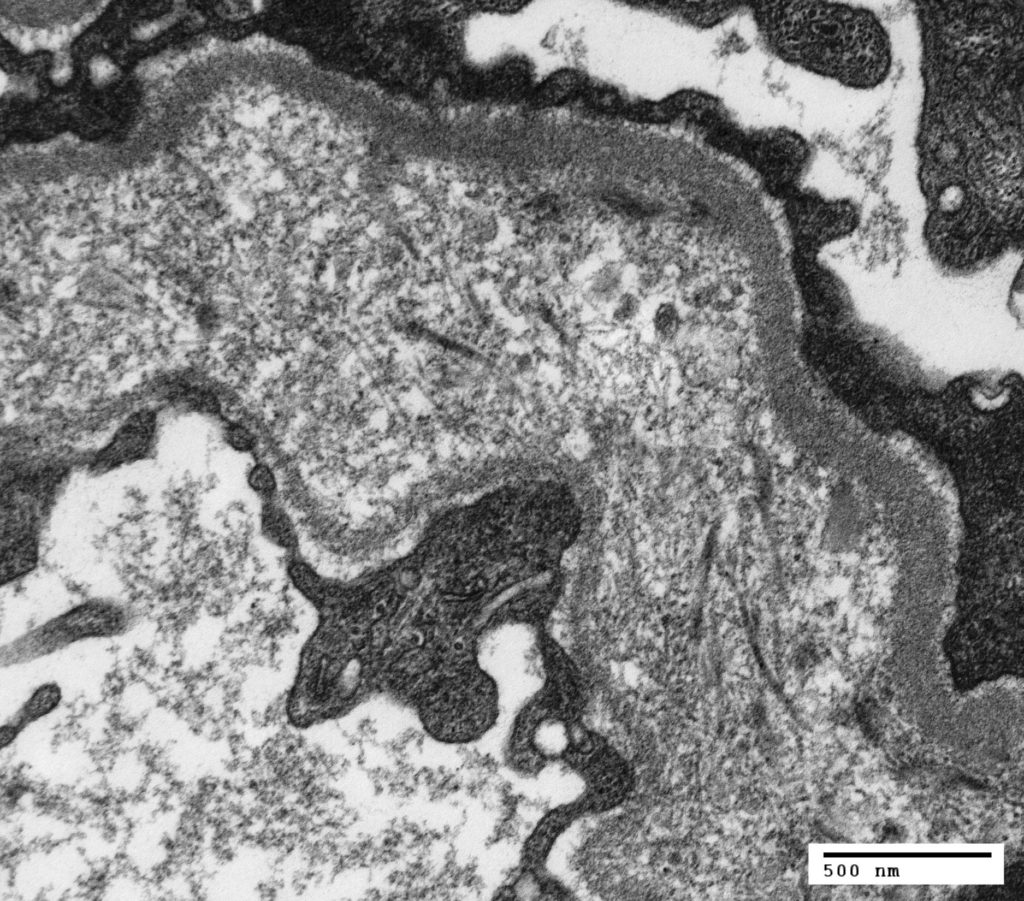
FIG.1E (TEM): The glomerular basement membrane is markedly widened and contains variably sized fibrils and granular material. There is podocyte foot process effacement.
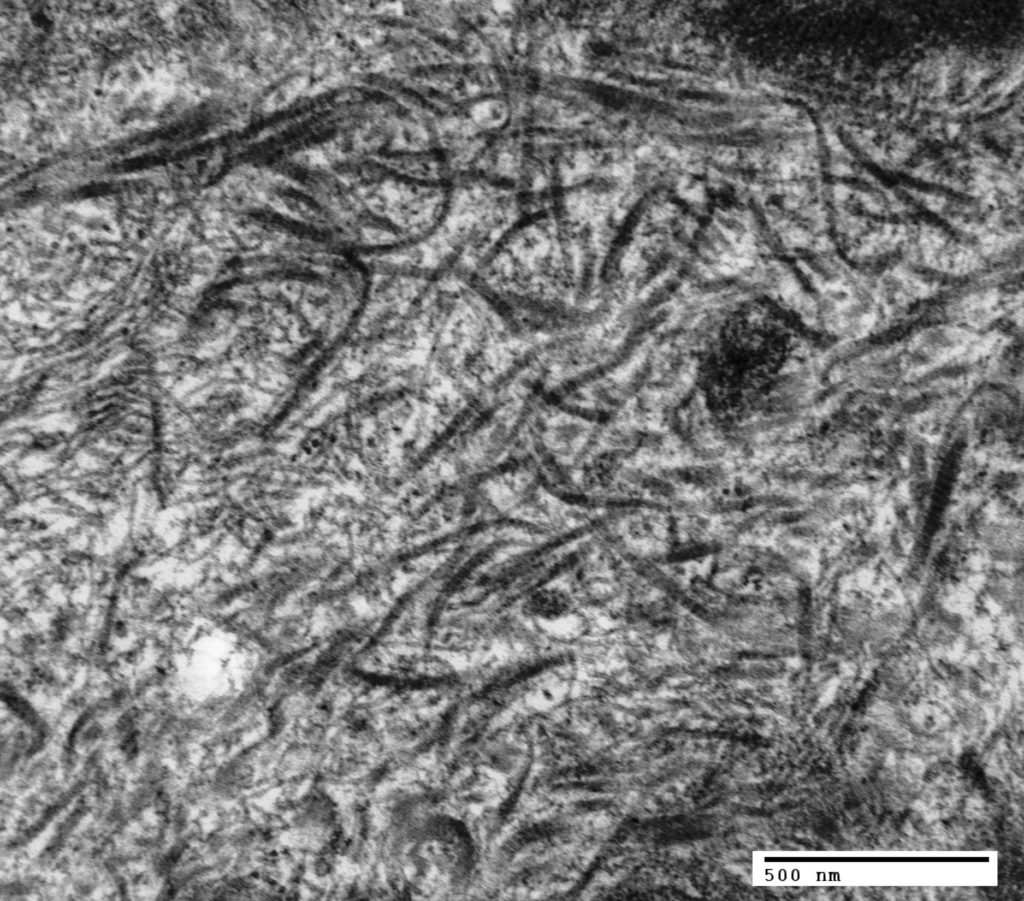
FIG.1F (TEM): The fibrils are often curvilinear and measure 10-33 nm in diameter. Prominent cross striations can be appreciated in larger fibrils.
ALPORT SYNDROME-LIKE NEPHROPATHY
- Alport nephropathy in humans is caused by an inherited defect in type IV collagen, which is a major component of glomerular basement membrane (GBM). Alport syndrome is caused by mutations in genes that encode the proteins for alpha-3 chain (COL4A3), alpha-4 chain (COL4A4), and/or alpha-5 chain (COL4A5) of type IV collagen, resulting in a structurally abnormal GBM and eventual glomerulosclerosis.
- JOCKD due to mutations in type IV collagen genes have been confirmed in few canine breeds (English cocker spaniel, English springer spaniel, Samoyed), as well as in a family of mixed-breed dogs from Navasota, Texas. Two different mutations in COL4A4 are responsible for the disease in English Cocker Spaniels and English springer spaniels, each of which is inherited as an autosomal recessive trait. Two different mutations in COL4A5 are responsible for the disease in Samoyeds and the Navasota dogs, each of which is inherited as an X-linked trait. These diseases often have been referred to as “Hereditary Nephropathy” (HN), autosomal recessive HN (ARHN), or X-linked HN (XLHN) in published reports.
- Light microscopic features are not specific for this disease. Glomerulosclerosis is common later in the disease, which may be misdiagnosed as MPGN based on light microscopy alone because the glomeruli can be hypercellular and the GBM is irregularly thickened.
Clinical Features
- Alport syndrome-like nephropathy is initially characterized by marked proteinuria prior to 6 months of age (Lees 2013).
- An increase in UPC is typically first observed between 4-6 months of age; if the dog is monitored closely, microalbuminuria can be identified prior to an increase in UPC (as early as 2 months of age in some dogs).
- The UPC typically reaches as high as 10-20. Hypoalbuminemia is commonly observed, but subcutaneous edema and/or ascites is not expected.
- Mild microscopic hematuria may be observed.
- Males with X-linked hereditary nephropathy (XLHN) and both males and females homozygous for autosomal recessive hereditary nephropathy (ARHN) demonstrate a progressive decline in GFR, typically reaching end-stage renal disease by one year of age (range 6-24 months of age). Dogs >2 years of age are unlikely to have XLHN or homozygous ARHN (Lees 1999) .
- Clinical signs are generally absent until moderate azotemia is present, after which a rapid clinical decline (usually within 1-2 months) is often observed.
- Hypertension is not a common feature of the disease.
- Carrier females with XLHN typically develop proteinuria by 6 months of age, but their proteinuria is less severe (UPC usually <5), and they demonstrate a more slowly progressive disease. The majority of carrier females have a normal lifespan, often succumbing to a non-renal disease.
- XLHN and ARHN have been identified in English Cocker Spaniels, English Springer Spaniels, Samoyeds, and mixed breed dogs. Of these, it has been identified as a breed-wide problem only in English Cocker Spaniels, and a genetic test has largely eliminated the disease in that breed (Lees 1998; Jansen 1987)
- A similar GBM lesion has been identified in Bull Terriers and Dalmatians (largely in Australia), but mutations in Type IV collagen have not been found. Similarly, reports of Doberman Pinschers, Beagles, and Rottweilers with the GBM lesion typical for HN are available in the literature (Hood 1995).
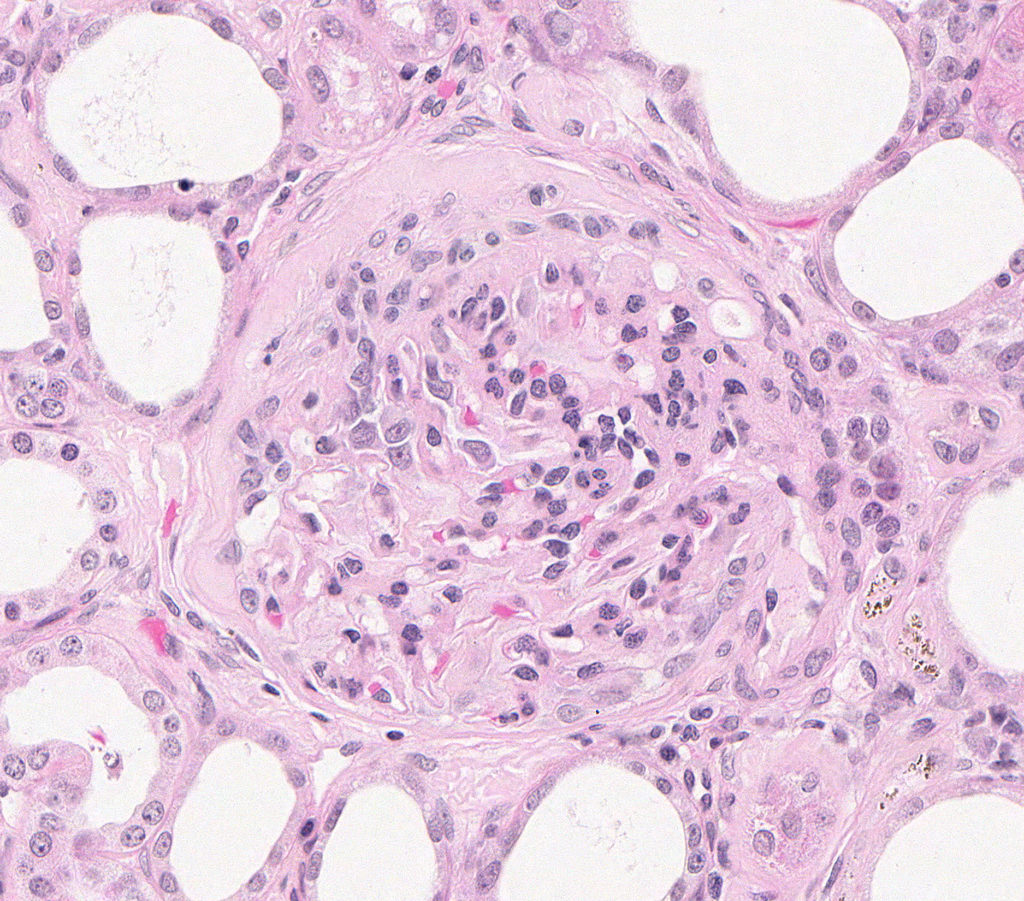
FIG.2A (HE): There is moderate to marked mesangial cell hypercellularity. A large portion of the capillary tuft is adhered to the thickened Bowman’s capsule. The surrounding interstitium is fibrotic.
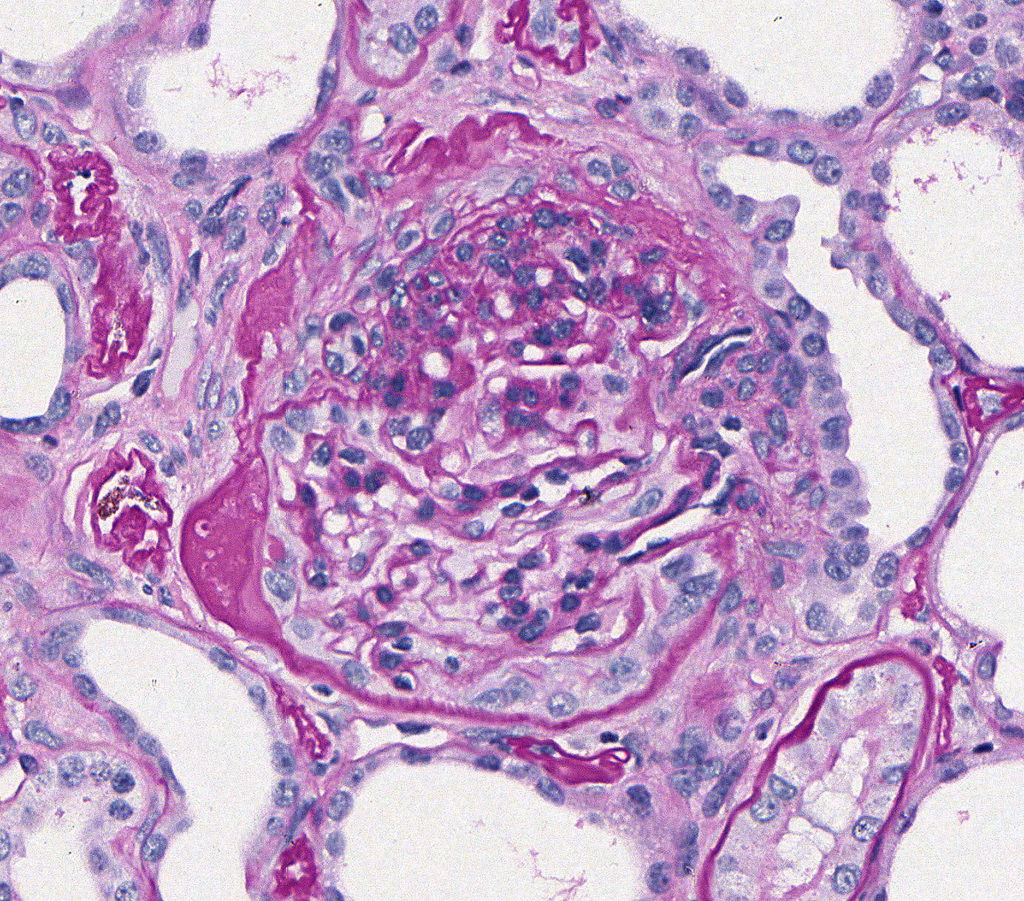
FIG.2B (PAS): There is segmental sclerosis of the capillary tuft, with adhesion of this portion to Bowman’s capsule (synechia). Note thickening, wrinkling, and cellular proliferation in area of synechia. There is podocyte hypertrophy and parietal epithelial cell hyperplasia.
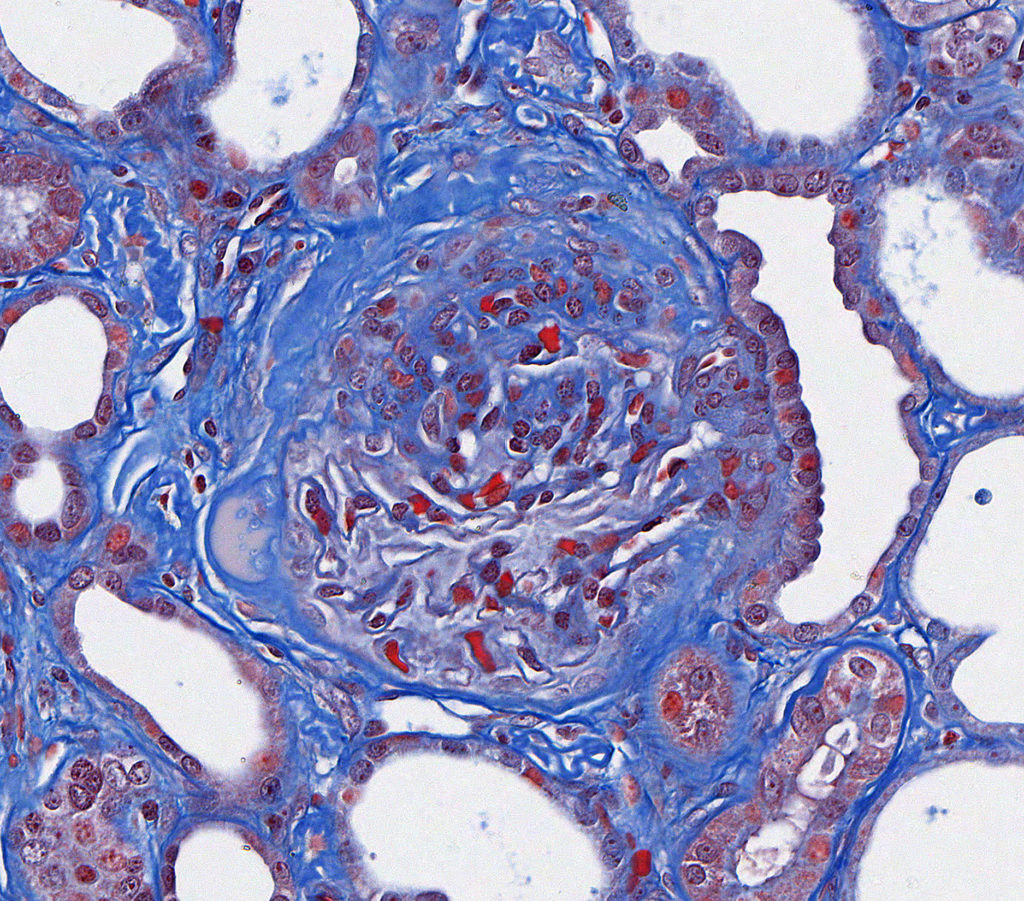
FIG.2C (MT): Segmental sclerosis with an extensive adhesion, wrinkling, and thickening of Bowman’s capsule, and fibrosis and mild inflammation in the adjacent interstitium.
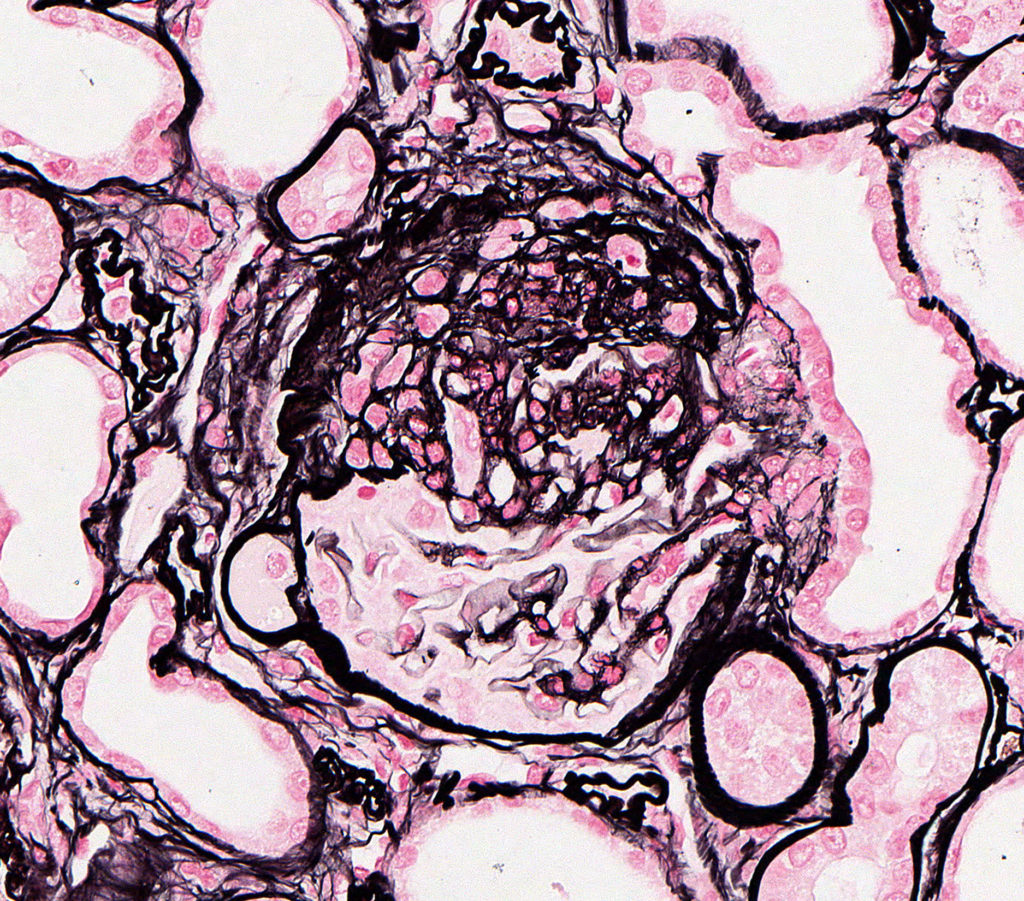
FIG.2D (JMS): Segmental sclerosis with an extensive adhesion, wrinkling, and thickening of Bowman’s capsule, and fibrosis and mild inflammation in the adjacent interstitium.
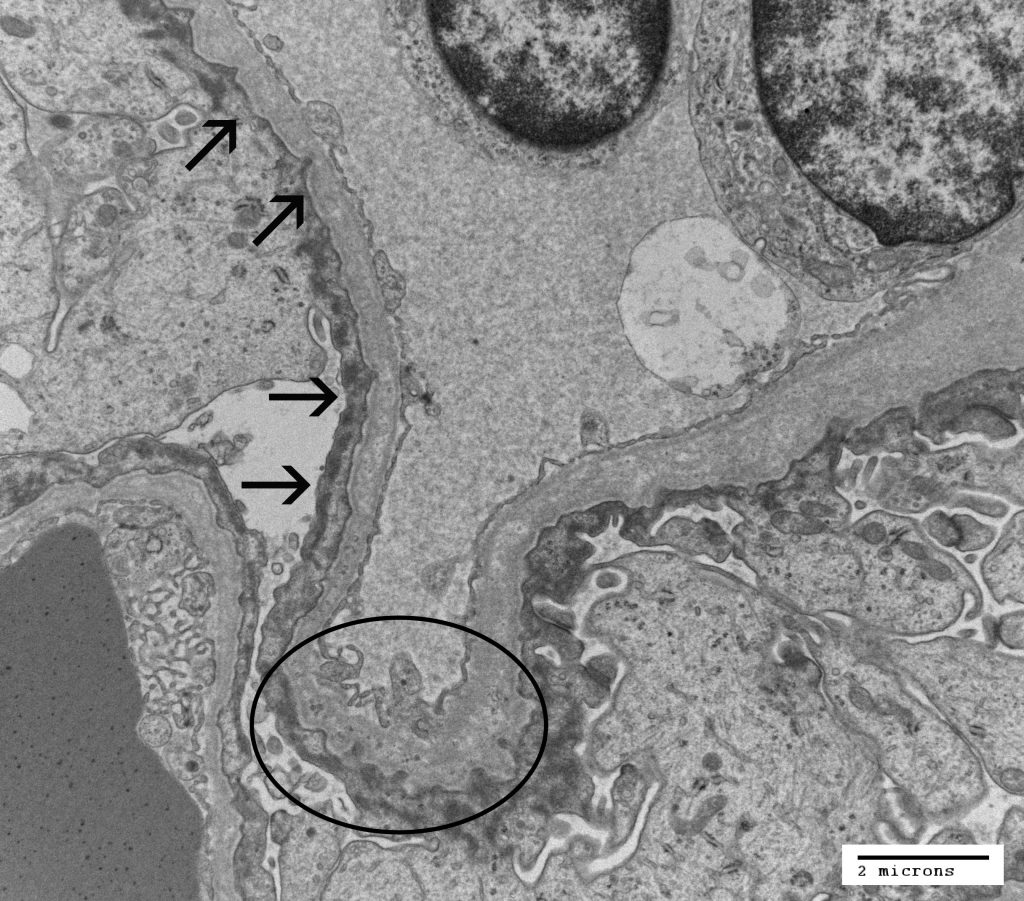
FIG.2E (TEM): Electron microscopy demonstrates the characteristic ultrastructural lesion of irregular GBM thickening and splitting. Other nonspecific changes include diffuse severe foot process effacement, reorganization of intracellular actin filaments (arrows), and irregularity of the abluminal GBM (circled).
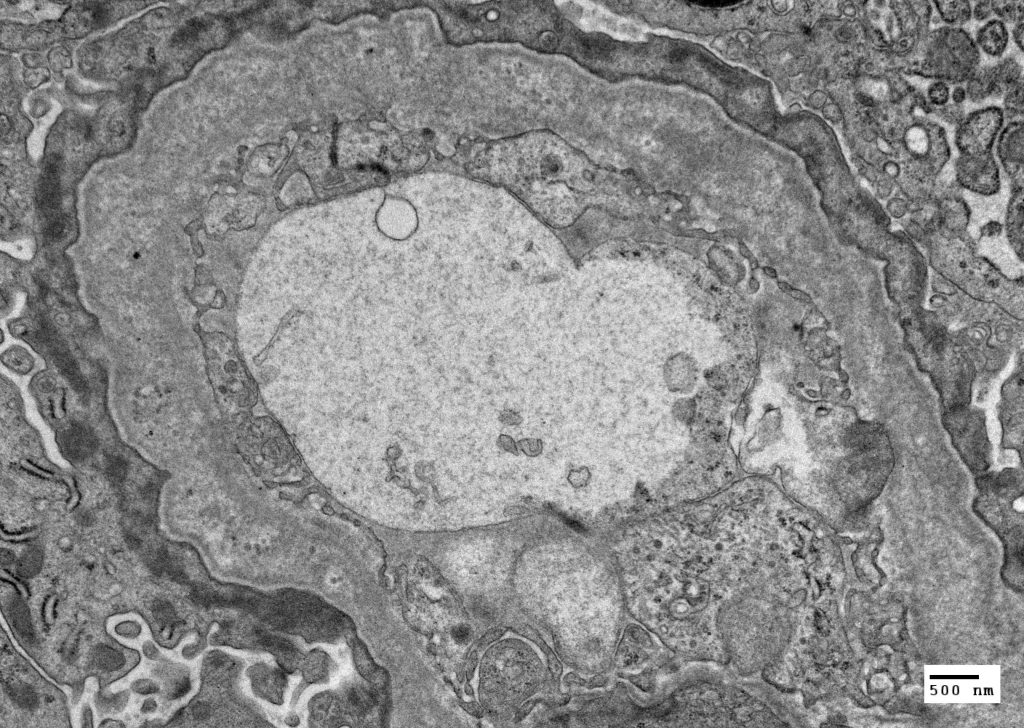
FIG.2F (TEM): The distinctive splitting, multilamination, and fragmentation of the lamina densa of the GBM is evident.
JUVENILE ONSET CHRONIC KIDNEY DISEASE DUE TO RENAL MALDEVELOPMENT
A short note on renal embryology is provided to illustrate the complex development of the kidney and to provide insight into patterns of malformation in the different compartments of the kidney. The mammalian kidney derives from the intermediate mesoderm of the urogenital ridge, a structure found along the posterior wall of the abdomen in the developing fetus. It develops in three successive stages known as the pronephros, the mesonephros, and the metanephros. In mammals, although the pronephros and mesonephros are required for renal development, they are transient structures which regress, allowing the metanephros or “metanephric kidney” to differentiate into the adult kidney. The completion of nephrogenesis in the dog can take up to two weeks post-natally (Eisenbrandt, 1979).
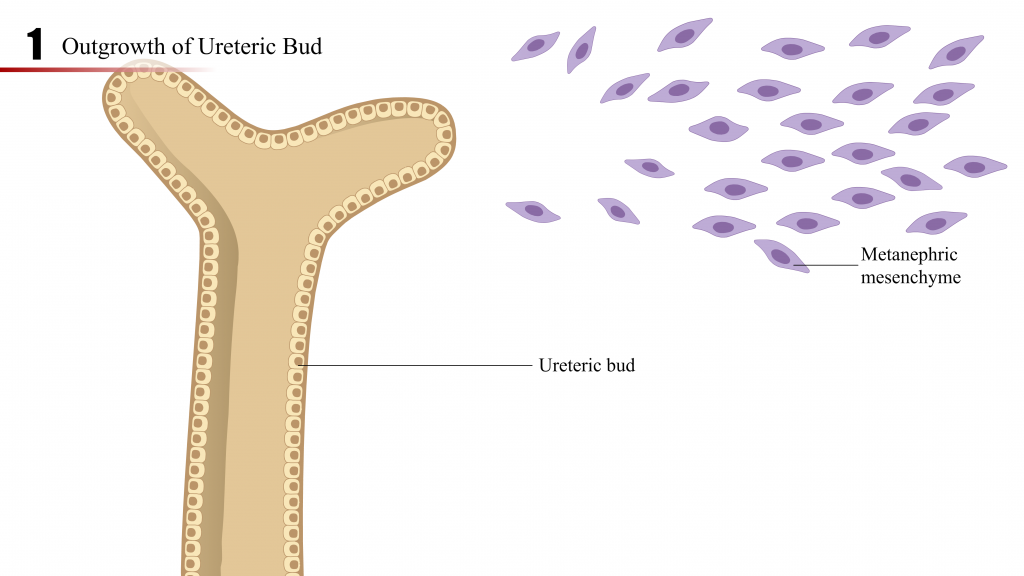
Nephrogenesis results from a series of inductive interactions between the metanephric mesenchyme (blastema) and the epithelial ureteric bud. Diagrams of the stages of nephron development are depicted below and an animation of the stages is also available. In the animation, the scrollbar below the image enables one to visualize the interaction between the ureteric bud at the metanephric mesenchyme.
-
- The epithelial-lined ureteric bud, an outgrowth of the distal end of the mesonephric duct, invades into the surrounding mesenchyme, and undergoes reiterative cycles of elongation, bifurcation, and differentiation to eventually form the collecting ducts, renal pelvis, and ureter by a process referred to as branching morphogenesis.
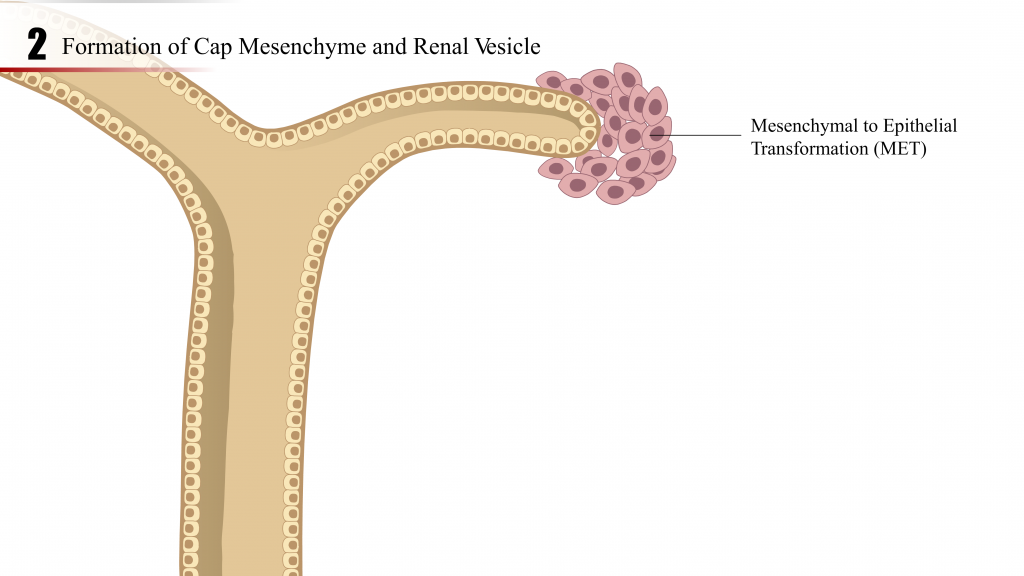
- The metanephric mesenchyme, by reciprocal induction with the ureteric bud, condenses, forms cellular aggregates, and undergoes mesenchymal – to – epithelial transition to become the renal vesicle.
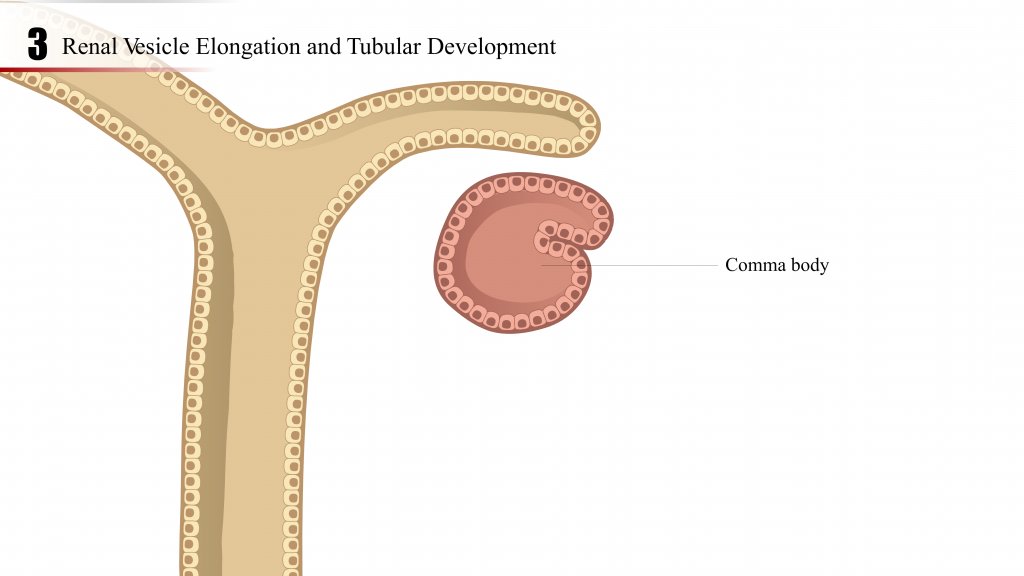
- The renal vesicle elongates and continues development through the comma-shaped body, S-shaped body, capillary loop and matures into the precursor of the nephron (tubules and glomerulus).
- Podocyte progenitor cells, first identified in the S-shaped body stage, attract the in-growth of endothelial cells. Capillary branching and mesangial cell recruitment and differentiation proceed until glomerular tuft formation is complete (glomerulogenesis).
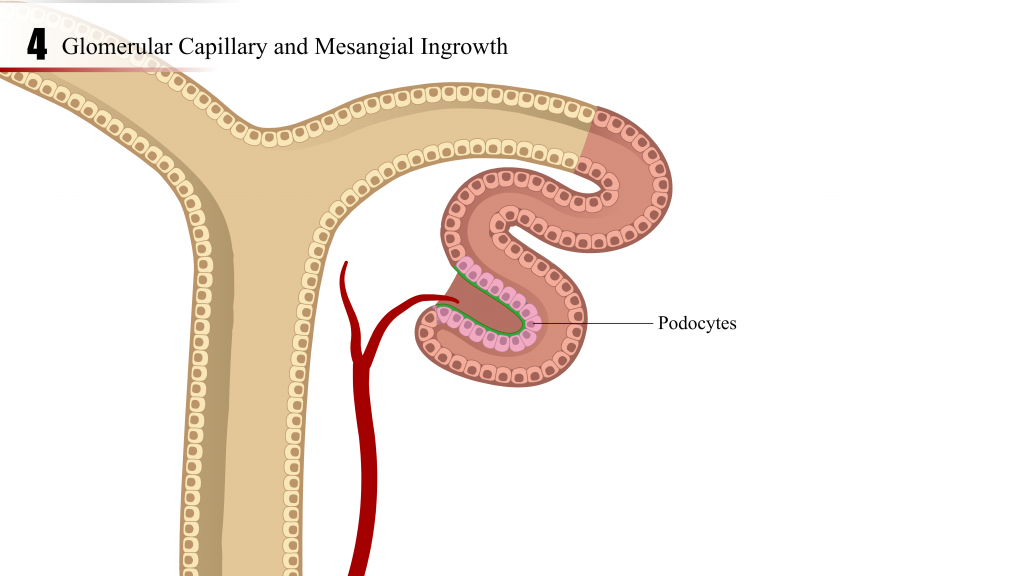
- The tubular portion of the nephron elongates while the glomerular capillary bed arborizes to form the final structure.
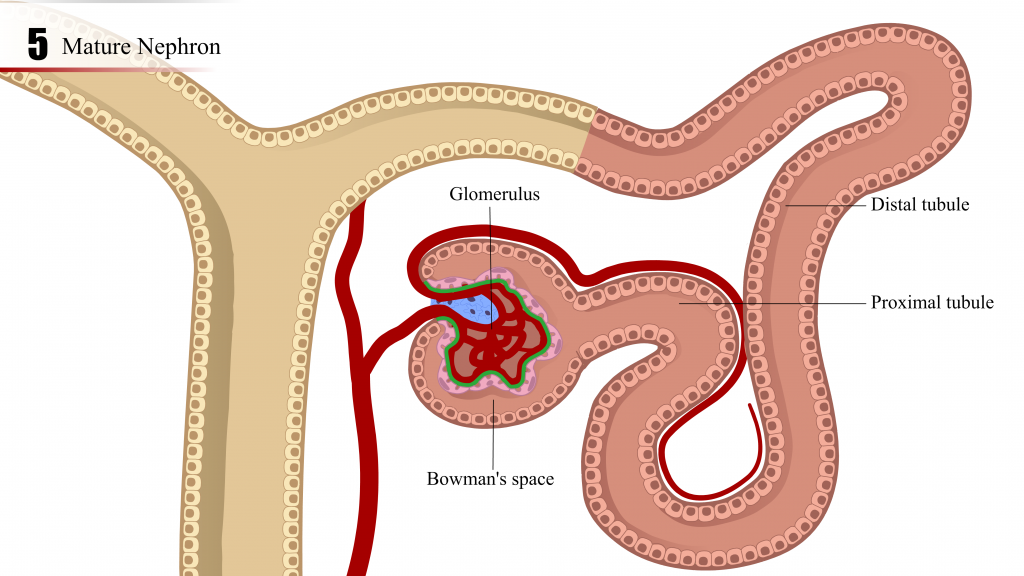
- The epithelial-lined ureteric bud, an outgrowth of the distal end of the mesonephric duct, invades into the surrounding mesenchyme, and undergoes reiterative cycles of elongation, bifurcation, and differentiation to eventually form the collecting ducts, renal pelvis, and ureter by a process referred to as branching morphogenesis.
In some species, including dogs, nephronogenesis and development continue during the post-natal period, reportedly up to 2 weeks after birth. The neonatal kidney from a 5 day old dog (Figure 3) has a prominent subcapsular zone of ongoing glomerular tuft development and tubular elongation.
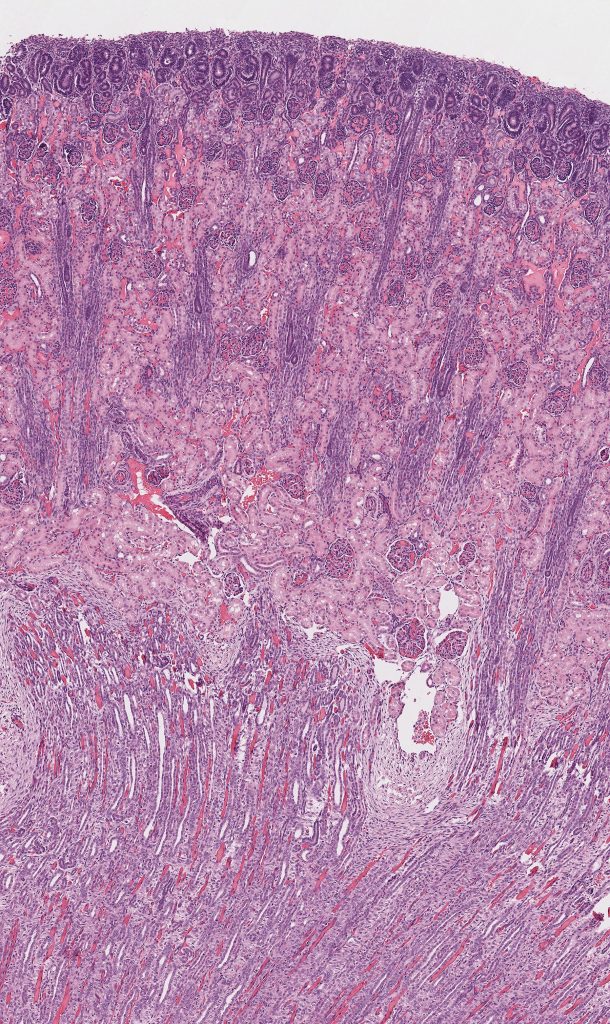
FIG.3A (HE): Beneath the renal capsule there is a blueish zone due to the crowded nuclei and small amounts of cytoplasm.
FIG.3B (HE): Higher magnification of Fig.3A. The subcapsular cortex contains metanephric mesenchyme and vesicles, with glomerulogenesis and nephron maturation progressing towards the outer subcapsular cortex. Note the S-shaped body (rectangle) with developing podocytes. The immature glomerulus has capillaries, although the podocytes are still arranged in a prominent row around the periphery of the tuft (arrows).
- Importantly, disrupted, disorganized and inadequate interactions between the metanephric mesenchyme, ureteric bud and stroma may result in renal maldevelopment.
- Renal hypoplasia is when the kidneys are smaller than expected but all of the components are mature and the architecture is normal.
- Renal maldevelopment is a type of JOCKD but they are not synonomous. Renal maldevelopment refers to disorganized development of renal parenchyma either in utero or during the neonatal period. Of note, the term “renal dysplasia” is deeply embedded in the veterinary lexicon; however, when used as a clinical diagnosis, renal dysplasia can be misconstrued by clinicians and animal owners as if it designated a single, specific disease entity. In our opinion, renal dysplasia should be viewed as a broad descriptive term for a general category of somewhat diverse disorders that are characterized by mostly developmental anomalies and/or lesions. We prefer to use “renal maldevelopment” as the diagnostic term for this category of disorders (ie, rather than renal dysplasia), because we believe that properly defining a new term will be more effective than attempting to re-define an old, sometimes misused, term.
- Renal maldevelopment is characterized at the light microscopic level by the presence of structures in the kidney that are inappropriate for the stage of development of the animal. The most frequent finding in renal maldevelopment are fetal / immature glomeruli and tubules located within radiating segments extending from the subcapsular surface to the corticomedullary junction (indicating asynchronous differentiation of nephrons). There also might be regions with a paucity of tubules and glomeruli; these foci instead contain non-inflamed connective tissue and often tortuous arterial profiles. Additional less common lesions of renal maldevelopment that are reported in dogs include: persistent mesenchyme, persistent metanephric ducts, atypical tubular epithelium, and dysontogenic metaplasia (presence of bone or cartilage in the renal parenchyma). Unfortunately, other than dysonotogenic metaplasia (which is a lesion that we have not personally observed within our own case material), there is not a consensus among pathologists regarding succinct definitions for these uncommon lesions. In fact, some lesions might even be acquired as opposed to be congenital. Specifically, in our experience, we have seen metanephric ducts and atypical tubular epithelium in adult dogs with varying underlying renal diseases (e.g. nephrolithiasis). Furthermore, the lesions listed above should not be considered pathognomonic for renal maldevelopment. For example, scattered fetal glomeruli are present in many adult small breed dogs and indicate arrested nephron development. Given the reserve capacity of the kidney, a few scattered immature nephrons are rarely clinically significant, and they can be identified in geriatric dogs with normal renal function. Therefore, diagnosis of renal maldevelopment is often a matter of degree, extent of the lesion, and the timepoint at which nephron development went awry.
- Secondary lesions in juvenile nephropathies include compensatory hypertrophy and hyperplasia of glomerular tufts and tubules, interstitial fibrosis, tubulointerstitial inflammation, pyelonephritis, dystrophic mineralization, cystic glomerular atrophy, microcystic tubules, retention cyst, and glomerular lipidosis.
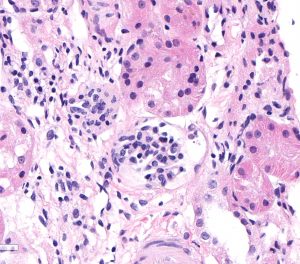
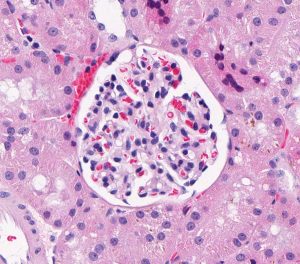
FIG.4A (HE): These 2 photomicrographs depict glomeruli from the same biopsy core taken at the same magnification. The left panel shows 2 fetal glomeruli which are small and poorly capillarized. The glomerulus in the center of the image has a mildly dilated Bowman’s capsule. The right panel shows a normal glomerulus.
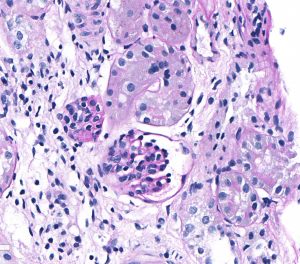
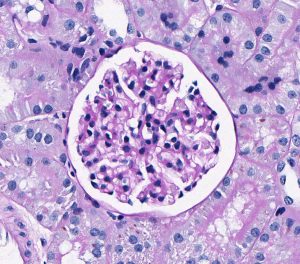
FIG.4B (PAS): Same glomeruli as depicted in above HE photomicrographs. It is easier to see the smaller fetal glomerulus in this stain because the PAS highlights the poorly expanded capillary network.
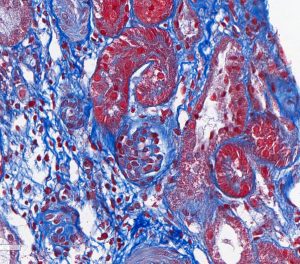
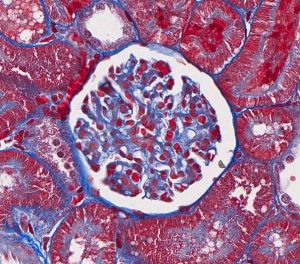
FIG.4C (MT): Same glomeruli as above. Because fetal glomeruli are small, one is not present in this section. This stain highlights the mildly inflamed interstitial fibrosis in this region with the fetal glomeruli, which is a common feature of maldevelopment.
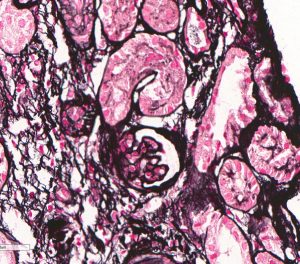
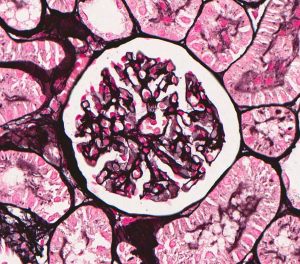
FIG.4D (JMS): Same glomeruli as above, again with only one fetal glomerulus in the left panel.
In addition to fetal glomeruli, dogs with renal madelevelopment might have large regions of dense collagen with minimal to mild inflammation and a paucity of tubular profiles.
FIG.5A (HE): Large region of dense collagen with numerous small, fetal or immature glomeruli (arrows). There is minimal inflammation, suggesting that the collagen is not secondary to previous inflammation or infection.
FIG.5B (PAS): The PAS stain is useful to highlight the numerous fetal or immature glomeruli (arrows). In the HE stain, they can often blend in with the background collagenous matrix.
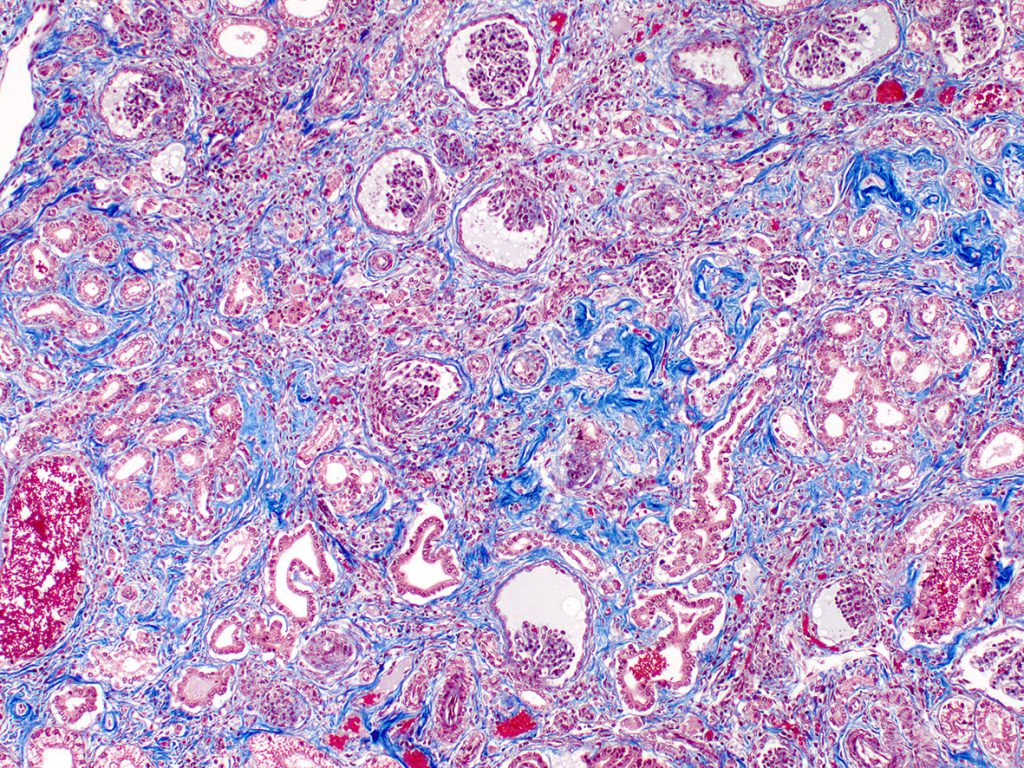
FIG.5C (TRI): The dense interstitial collagen stains dark blue.
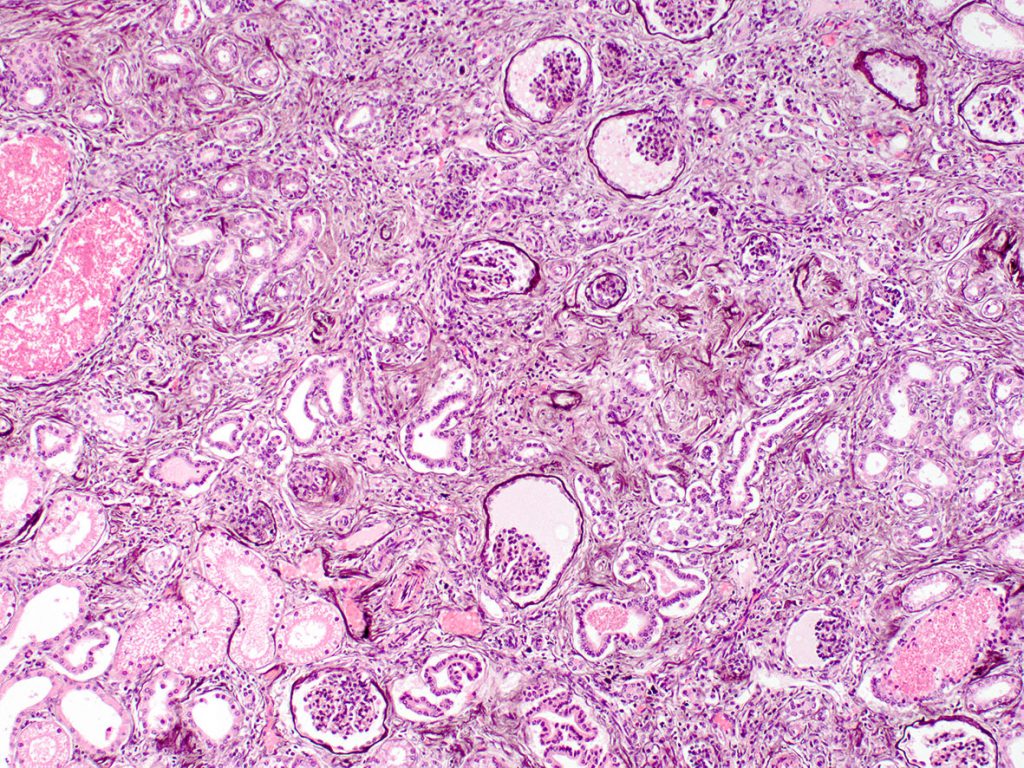
FIG.5D (PAMS): The silver stain highlights the basement membranes of the glomerulus, Bowman’s capsules and tubules.
In many cases, the region of maldevelopment has a wedge-shaped appearance radiating towards the renal capsule. These likely represent lobules or nephron units that did not develop normally and are immediately adjacent to normal renal parenchyma.
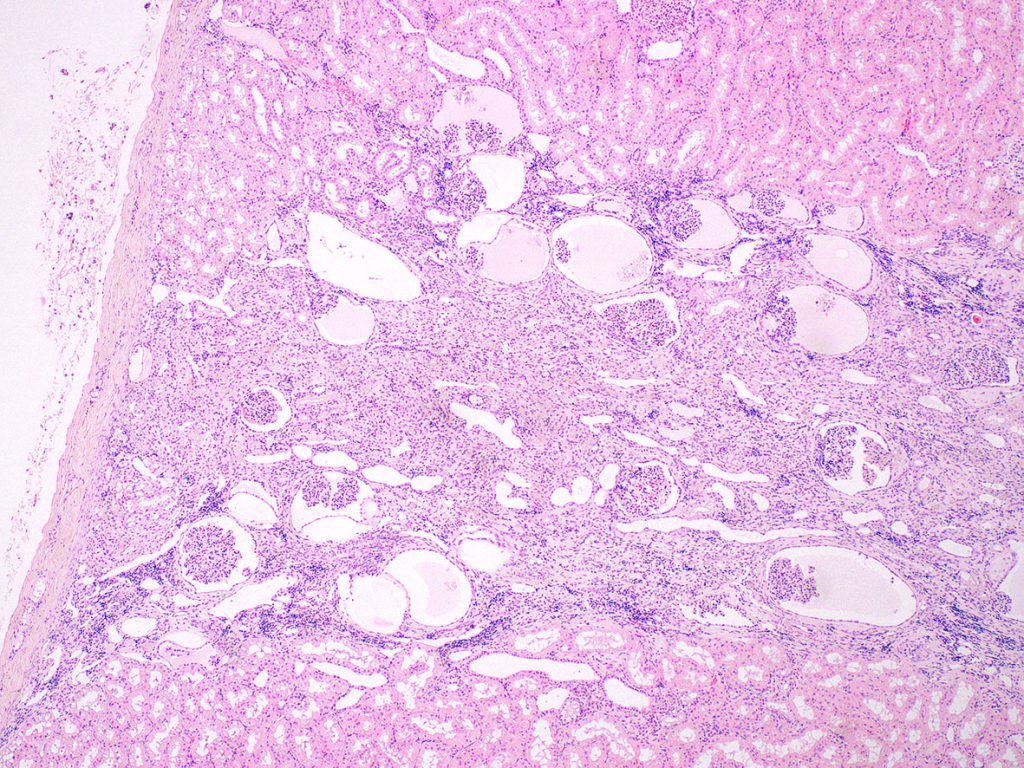
FIG.6A (HE): In this sample of renal cortex, there is a radiating streak of mildly inflamed interstitial fibrosis that separates the dilated ducts and glomeruli with cystic Bowman’s capsules.
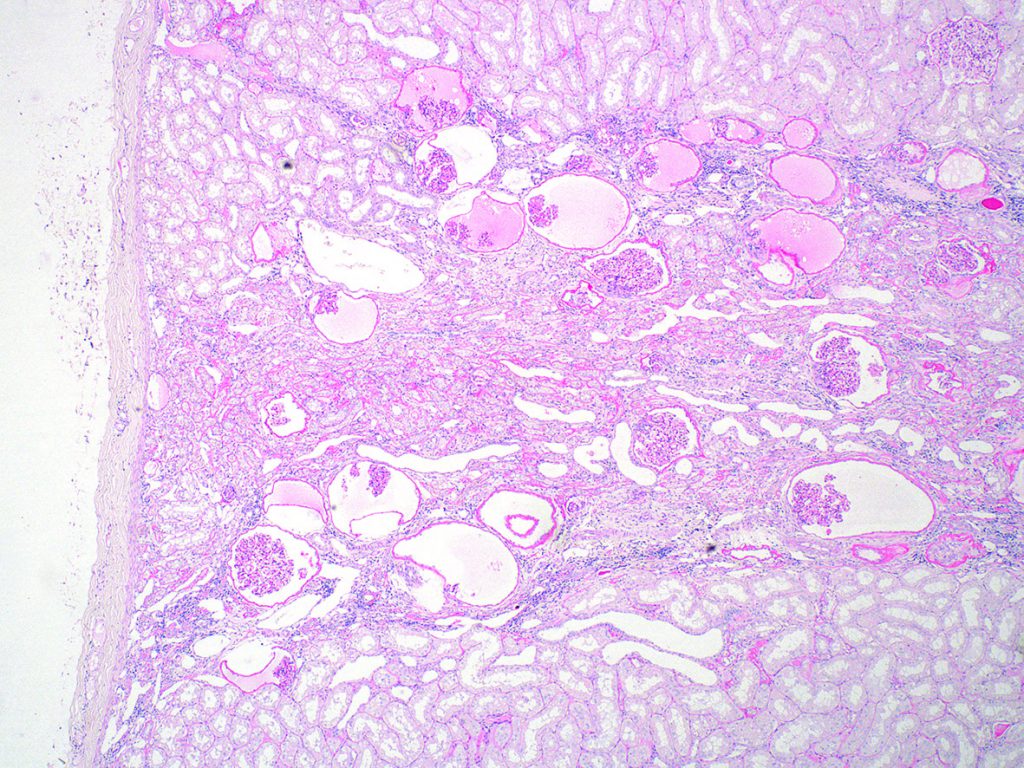
FIG.6B (PAS): Same region as above. This stain highlights the basement membranes of small tubular profiles.
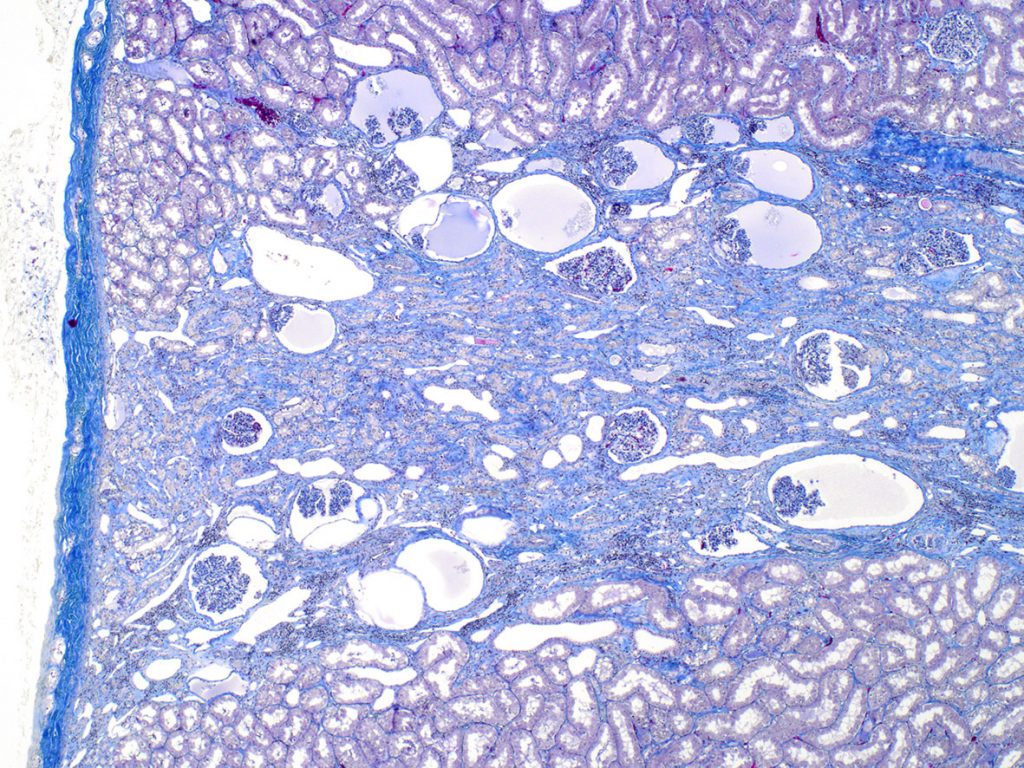
FIG.6C (MT): Same region as above. This stains highlights the interstitial fibrosis that separates the small tubular profiles.
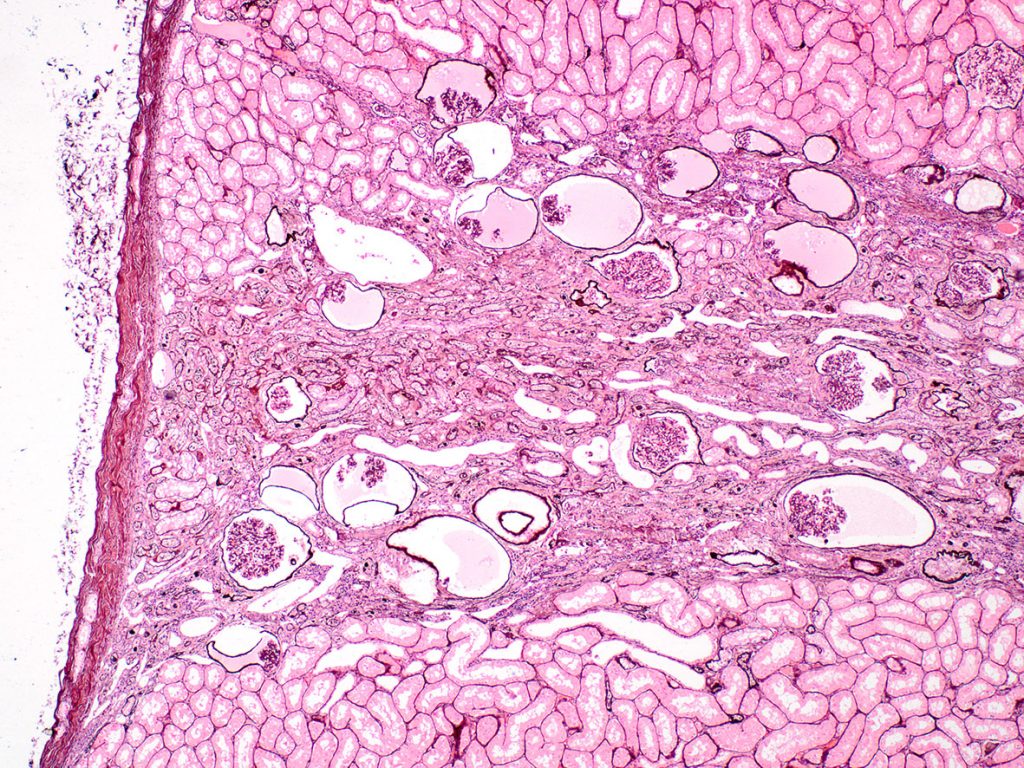 FIG.6D (PAMS): The basement membranes of the dilated Bowman’s capsules and tubular basement membranes stain prominently.
FIG.6D (PAMS): The basement membranes of the dilated Bowman’s capsules and tubular basement membranes stain prominently.
The wedge or lobular appearance can be easy to discern in nephrectomy or autopsy samples, but imagine how a needle core biopsy of the above kidney might appear. Regions of dense collagen will likely be harder to cut through, compared to the normal parenchyma. In our experience, core biopsies from animals with abnormally developed wedges or lobules will harvest only small portions of the collagenous tissue which are immediately adjacent to normal kidney tissue. Importantly, however, the fetal glomeruli in these pieces should be included in the overall count to estimate the proportion of non-functioning nephrons in the patient.
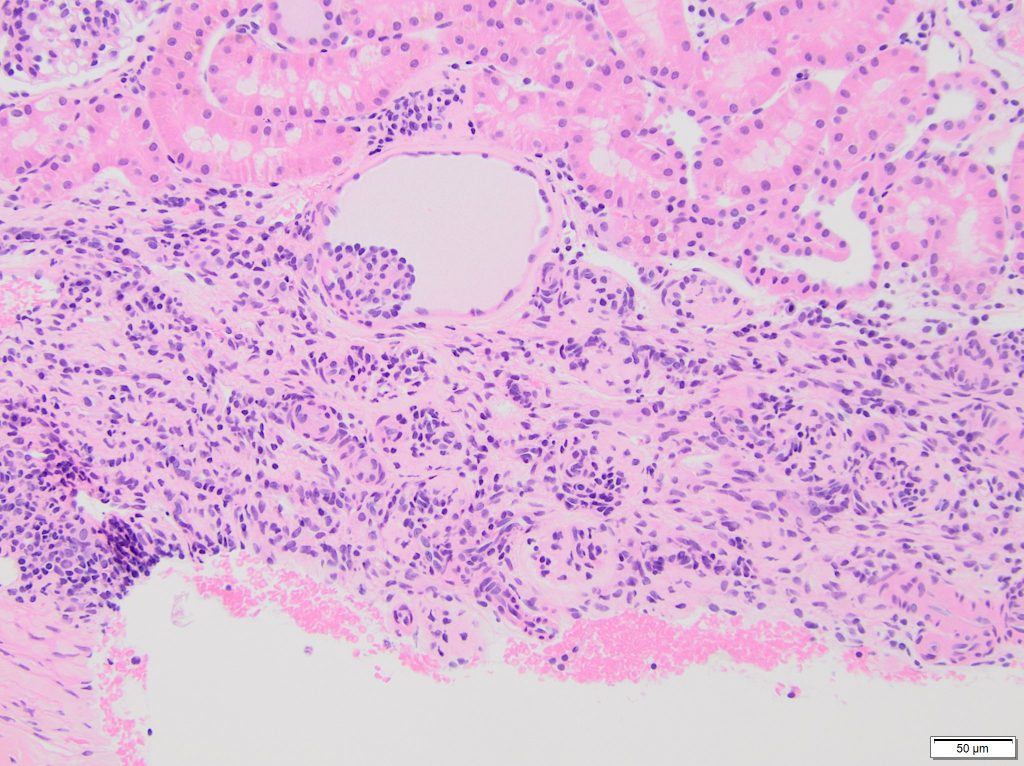
FIG.7A (HE): The HE stain shows a well-demarcated region of collagen and small aggregates of nuclei, which are the fetal glomeruli. One fetal glomerulus is contained within a moderately dilated Bowman’s capsule. The adjacent renal parenchyma appears normal.
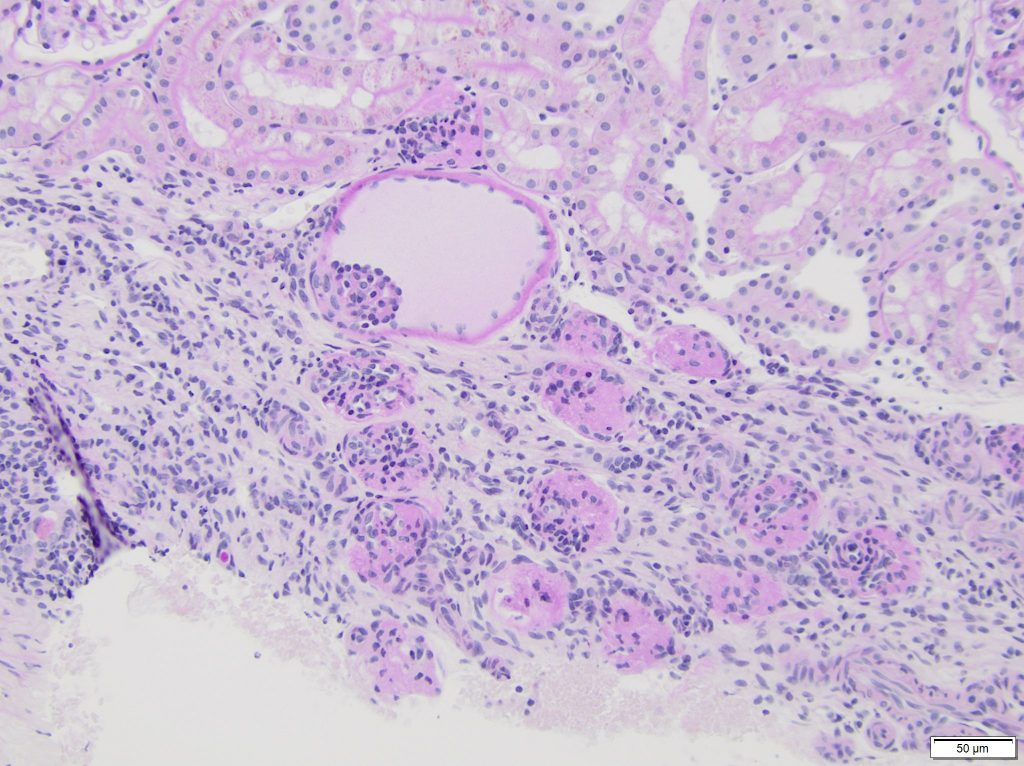
FIG.7B (PAS): The PAS stain highlights the numerous fetal glomeruli at the edge of a core biopsy. The adjacent renal parenchyma appears normal.
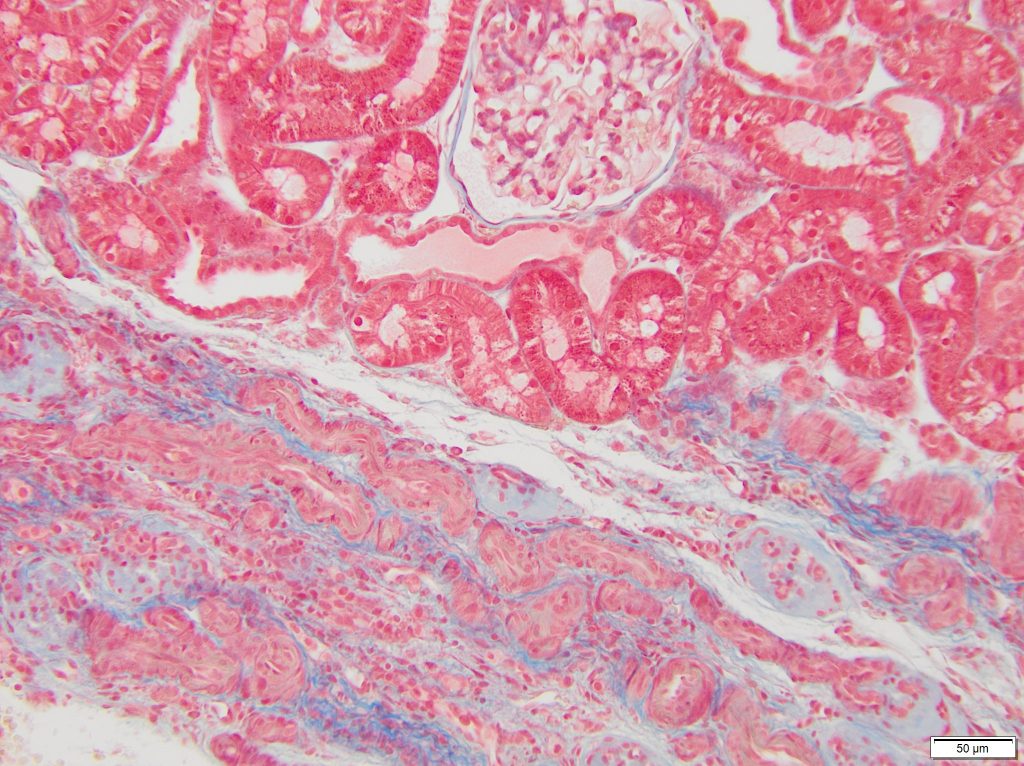
FIG.7C (MT): The fetal glomeruli are somewhat difficult to see because the collagen blends with the interstitium. However, multiple arterial profiles are close together, whereas they would normally be separated by glomeruli and tubular profiles. 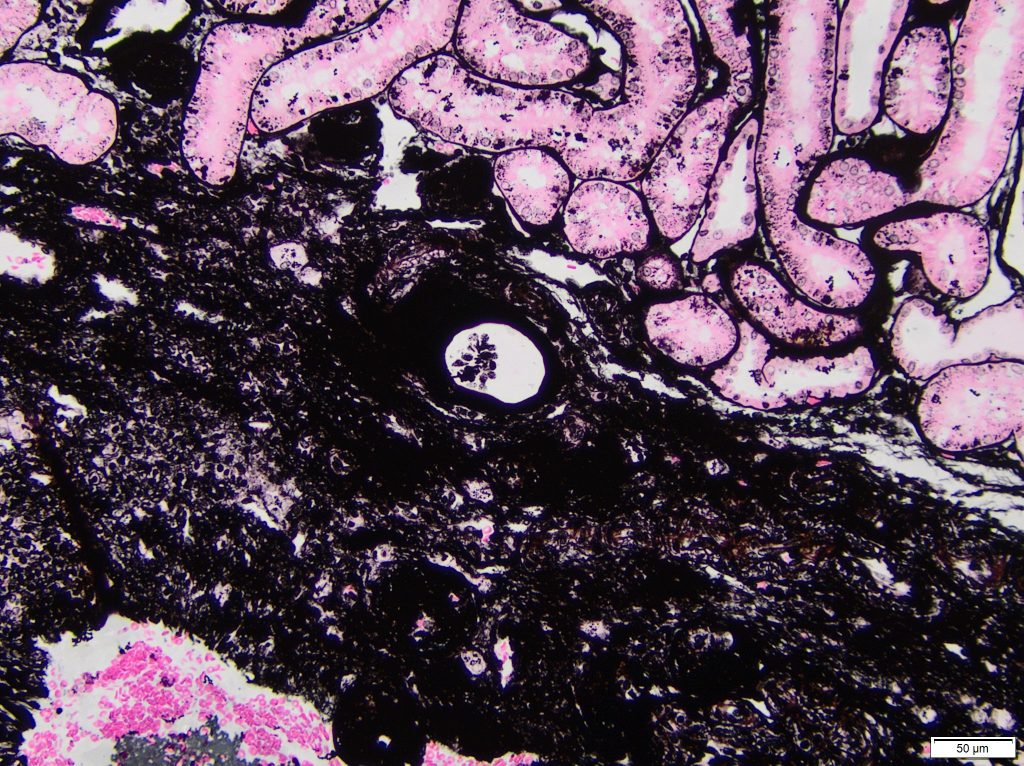
FIG.7D (JMS): The maldeveloped region stains dark black with the JMS stain. A single glomerular profile with a dilated Bowman’s capsule is evident at the junction between normal and abnormal parenchyma.
Other less common lesions of renal malevelopment include numerous cystically dilated tubules or ducts. These are unusual lesions in young animals with normal nephron development. Notably, however, diffuse tubular and ductal dilation can be acquired lesions secondary to obstruction. Therefore, examination of the papilla, renal pelvis and lower urinary tract is recommended to rule out obstructive nephropathy. Focal dilation can be secondary to intratubular / intraductal casts or from compression by the interstitium.
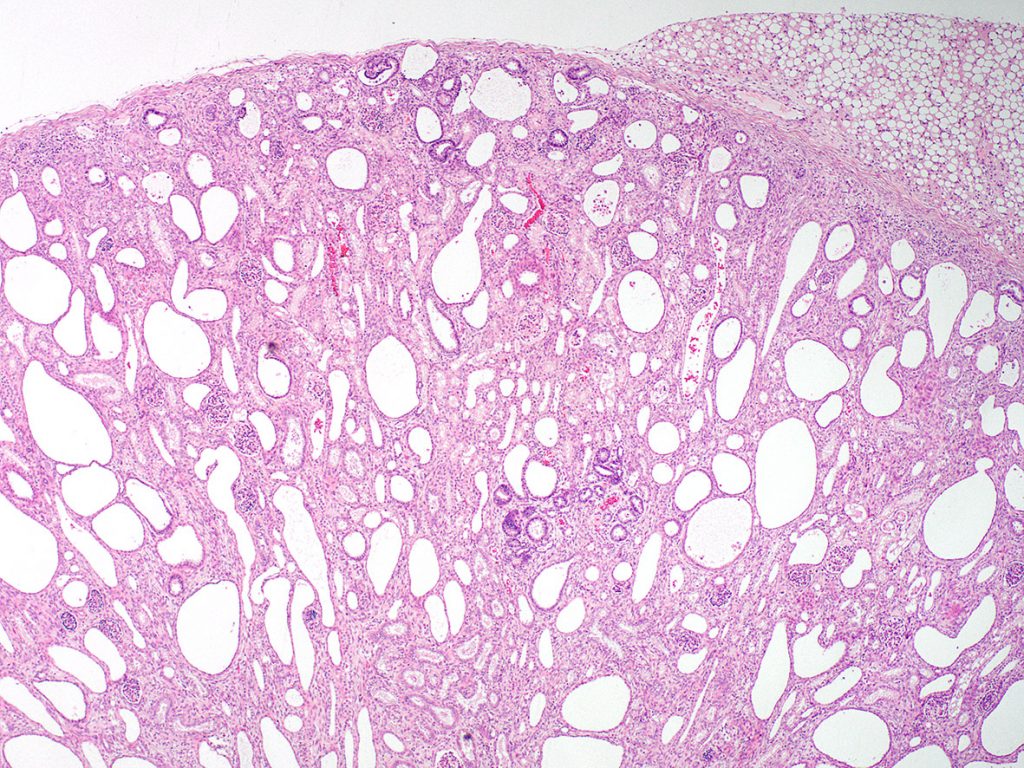
FIG.8A (HE): The entire cortical parenchyma is abnormal with numerous dilated tubular and ductal profiles. This appearance can be mistakenly called polycystic kidney disease (PKD); however, the diagnosis of PKD should be reserved for cases in which there are proven mutations in PKD-1 or PKD-2 genes. Cysts in PKD patients develop over time due to abnormalities in the primary cilia of tubular epithelial cells. Humans that have numerous cysts secondary to abnormal nephron development are diagnosed with “multicystic dysplasia”.
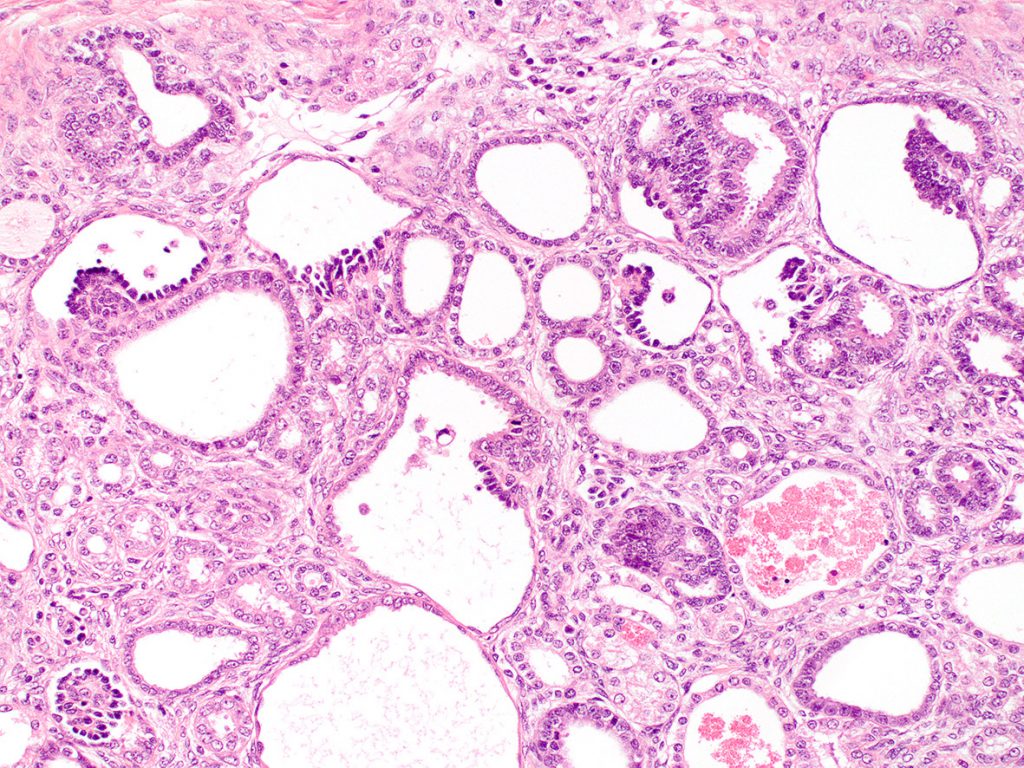
FIG.8B (HE): Higher magnification of the above photomicrograph shows that the dilated tubules and ducts are admixed with smaller tubules that have crowded columnar epithelium. There is also uninflamed fibrosis. A few tubules have red globular casts within their lumens. These casts are suggestive of fragmented red blood cells, hemoglobin or myoglobin pigments. 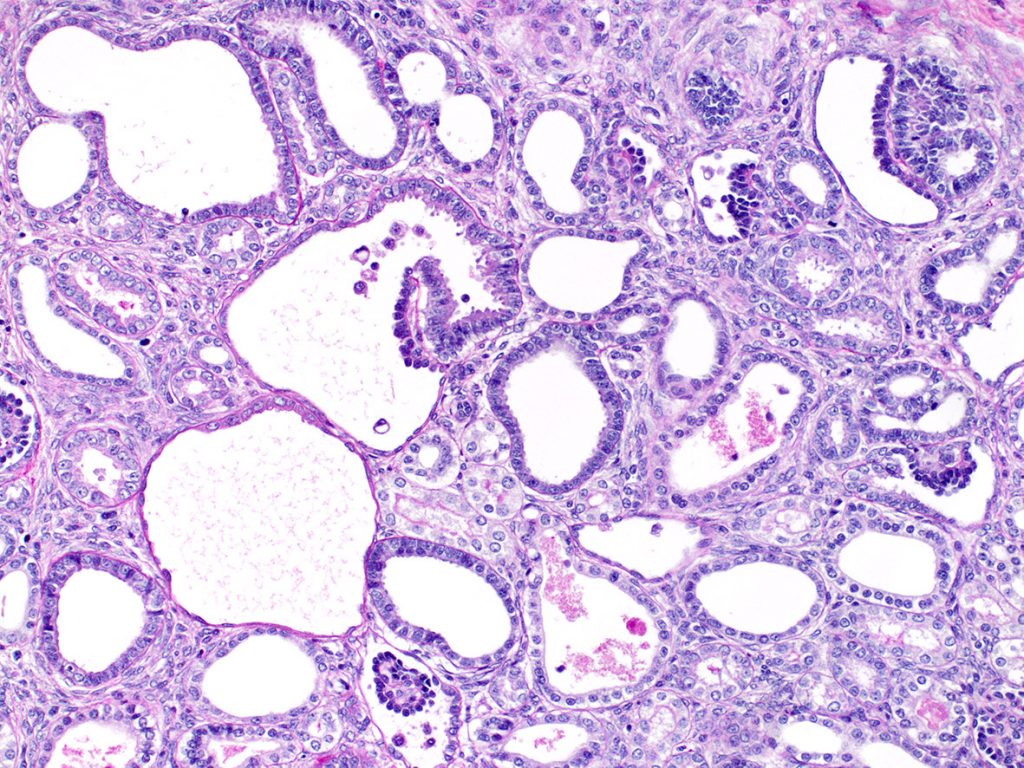
FIG.8C (PAS): This stain demonstrates that the dilated tubules and ducts lack a prominent apical brush border. There is one entire and one partial glomerulus in this photomicrograph.
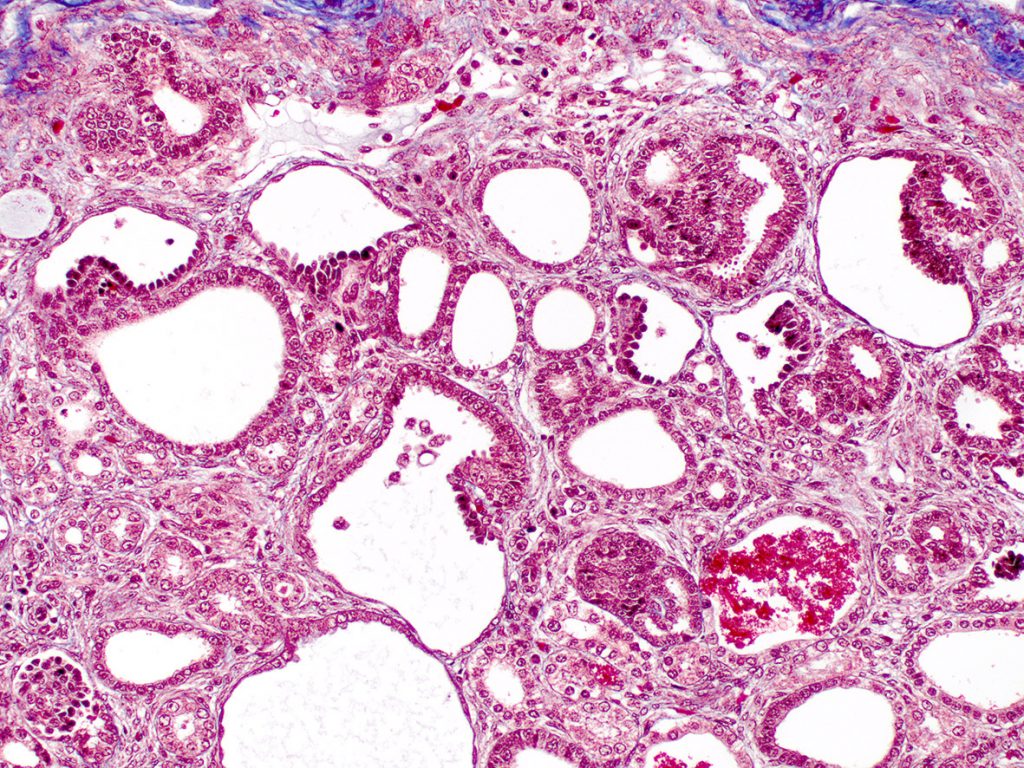
FIG.8D (MT): There is increased interstitial collagen in this specimen but it is not as dense as observed in Figure 5C. This might be a reflection of the difference in the age of the patients. The red globular casts that were seen in Figure 8B are bright red on with MT, suggestive of red blood cell fragments, hemoglobin or myoglobin.
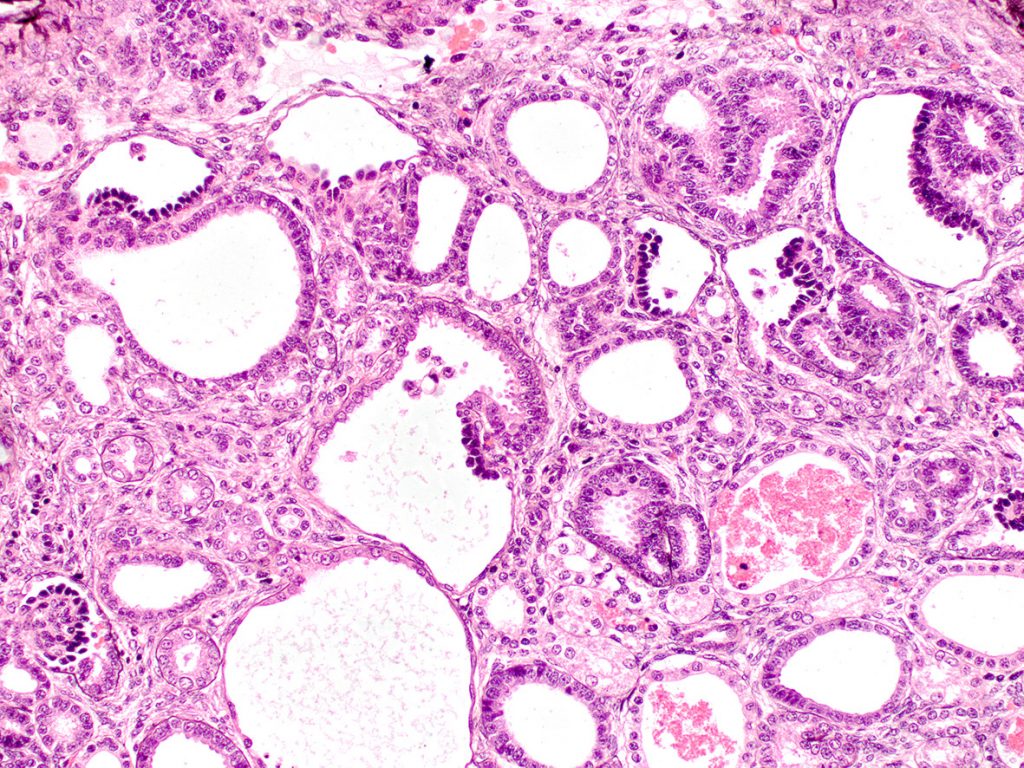
FIG.8E (PAMS): The tubular basement membranes are of normal thickness and do not have evidence of multi-lamination.
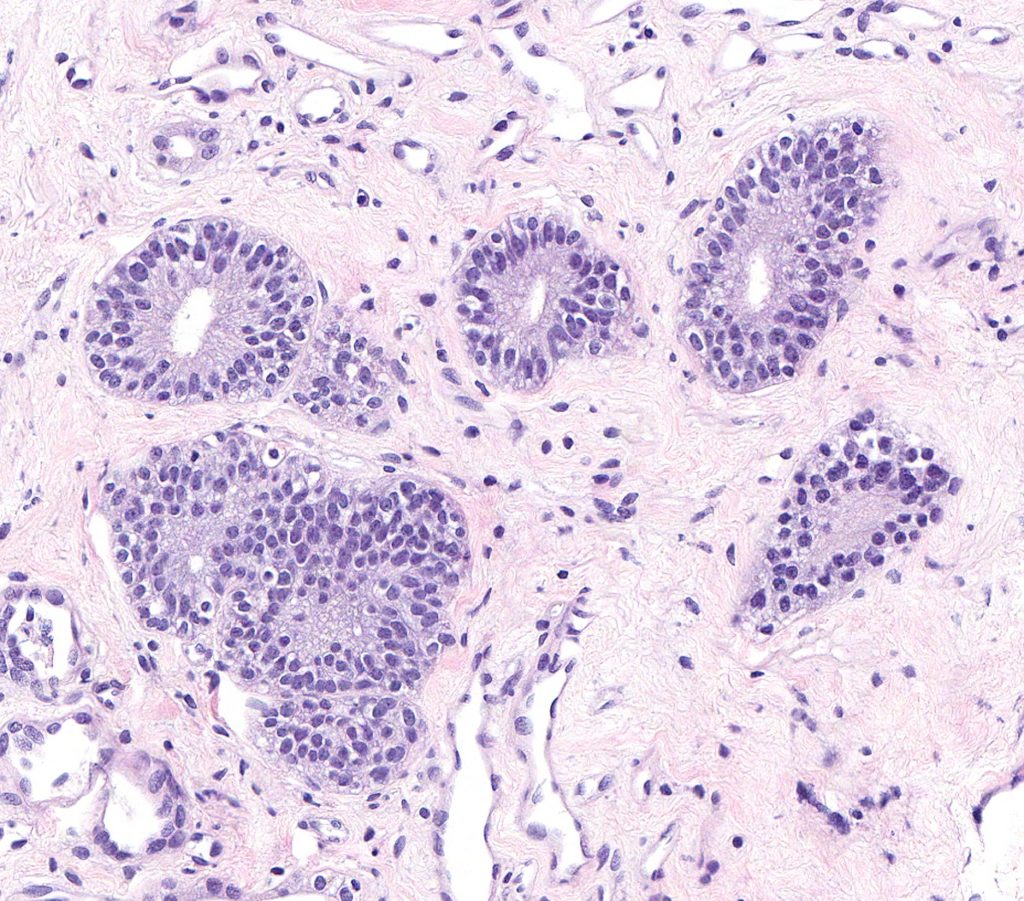
FIG.9A (HE): In samples that contain medullary tissue, ducts such as these might be identified. They are line by tall columnar to pseudostratified epithelium and are sometimes called “metanephric ducts”.
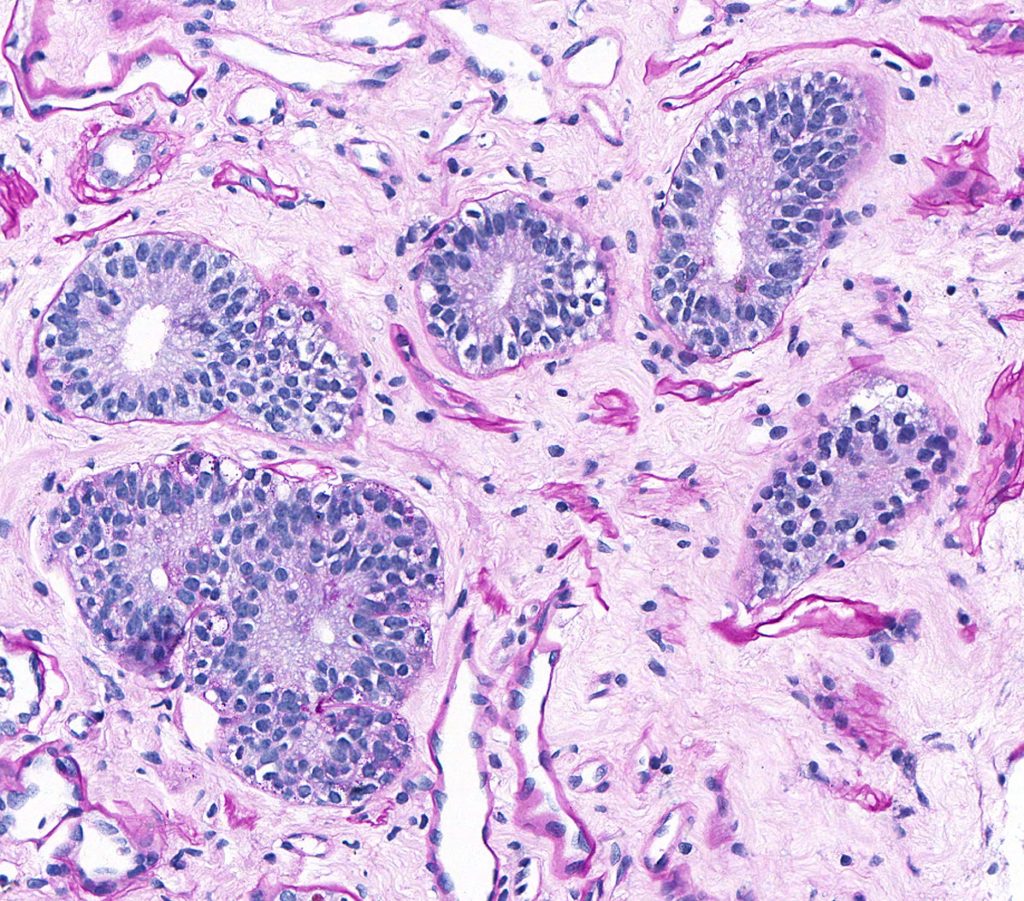
FIG.9B (PAS): This stain shows the ducts with the tall columnar epithelium but there are also admixed tubular profiles with cuboidal to attenuated epithelium (segments of Loops of Henle).
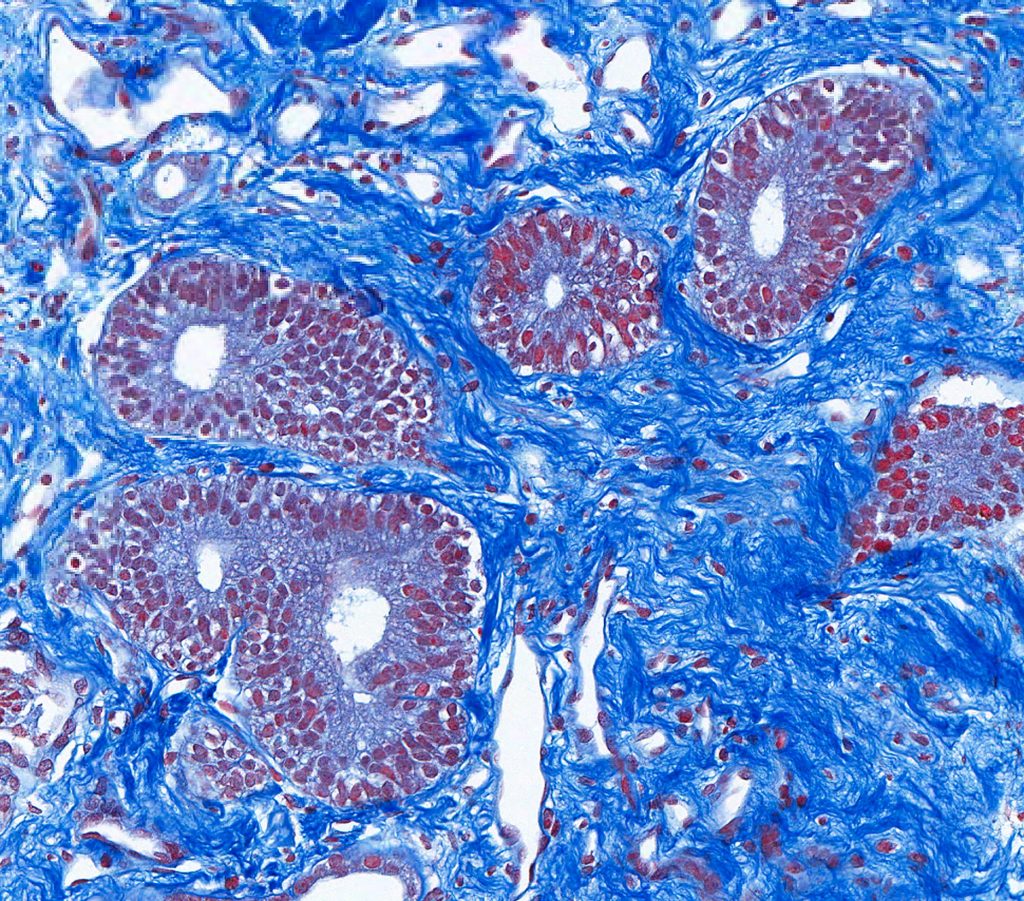
FIG.9C (MT): There is usually increased collagenous matrix in the medullary interstitium in normal kidneys. This should not be diagnosed as papillary or medullary fibrosis.
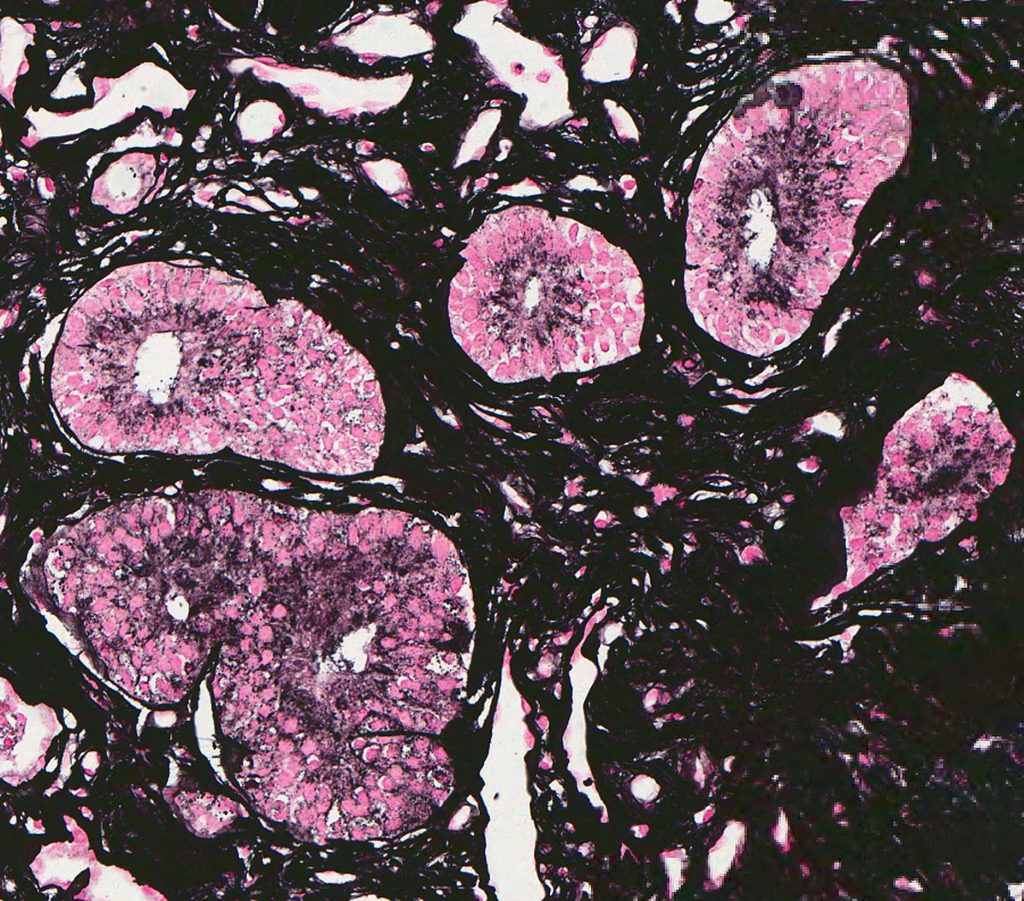
FIG.9D (JMS): The same region as above.
In some breeds, as well as in some individual dogs, the histopathology can be dominated by the presence of small compressed glomerular tufts and severely dilated Bowman’s capsules (glomerulocystic atrophy). This phenotype has been reported in the French bull mastiff but has also been observed in Boxer dogs. In humans, the lesion is sometimes diagnosed as Glomerulocystic Kidney Disease.
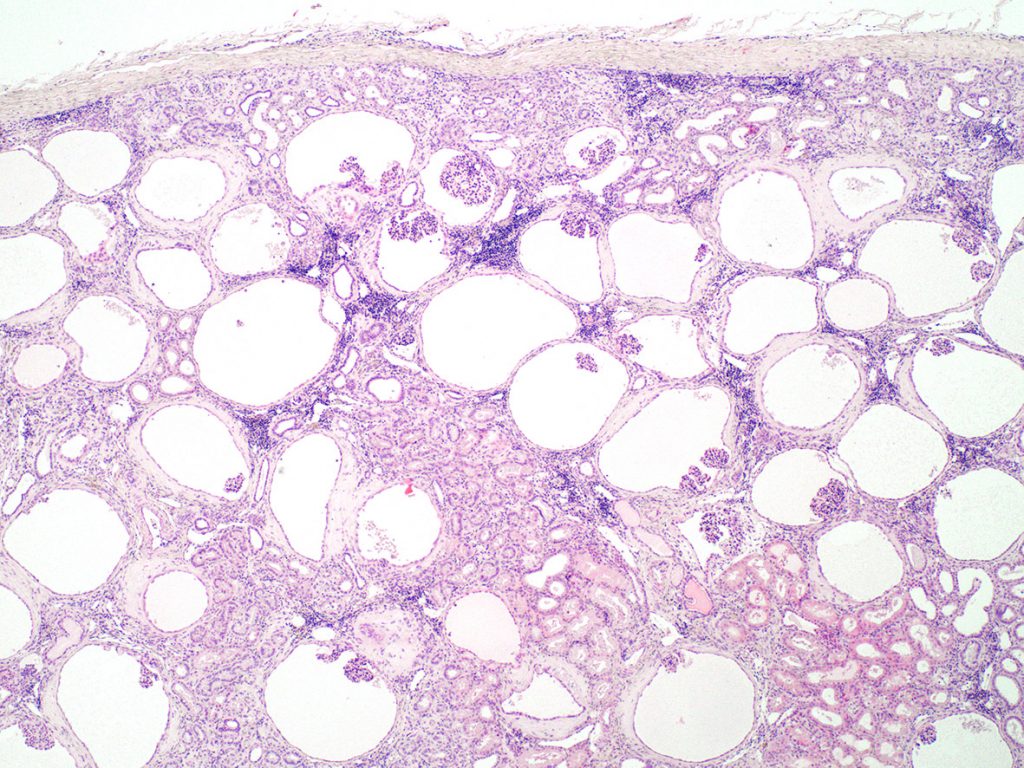
FIG.10A (HE): Most of the glomeruli are small compressed tufts within dilated Bowman’s capsules. The clear circles are also dilated Bowman’s capsules but the capillary tuft is so small that it is not present in the section. There is mild periglomerular inflammation likely due to small amounts of leakage of the urinary filtrate into the periglomerular interstitium. The tubular profiles are relatively normal.
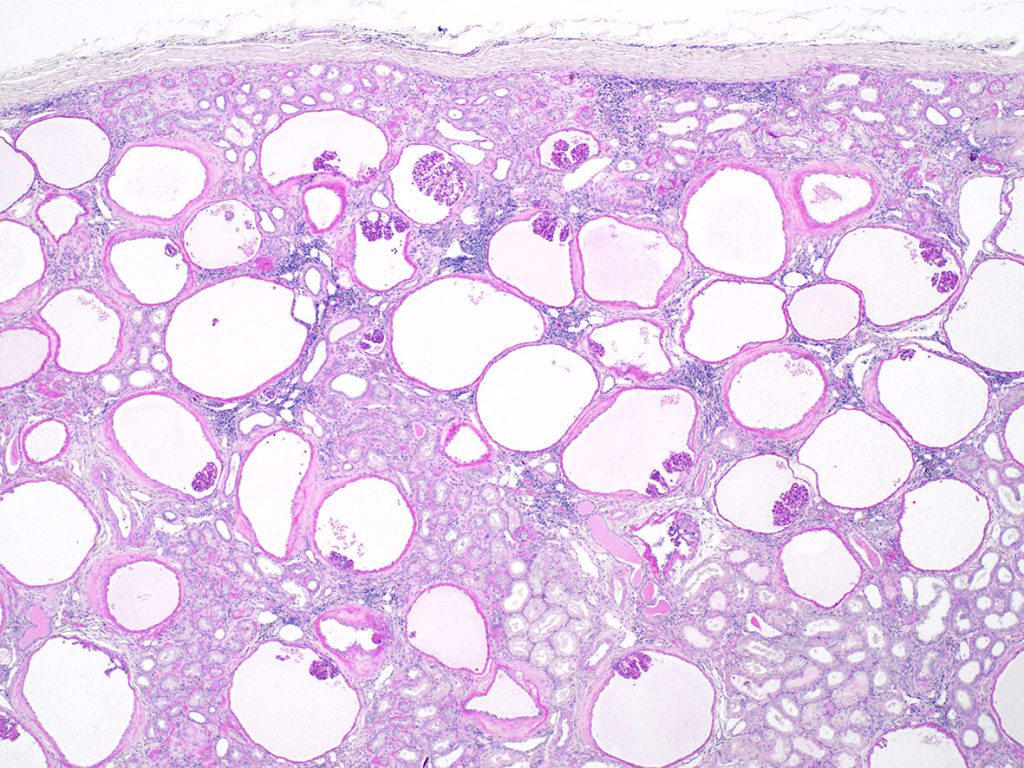
FIG.10B (PAS): The atrophic glomerular tufts stain dark pink and capillary lumens are difficult to appreciate.
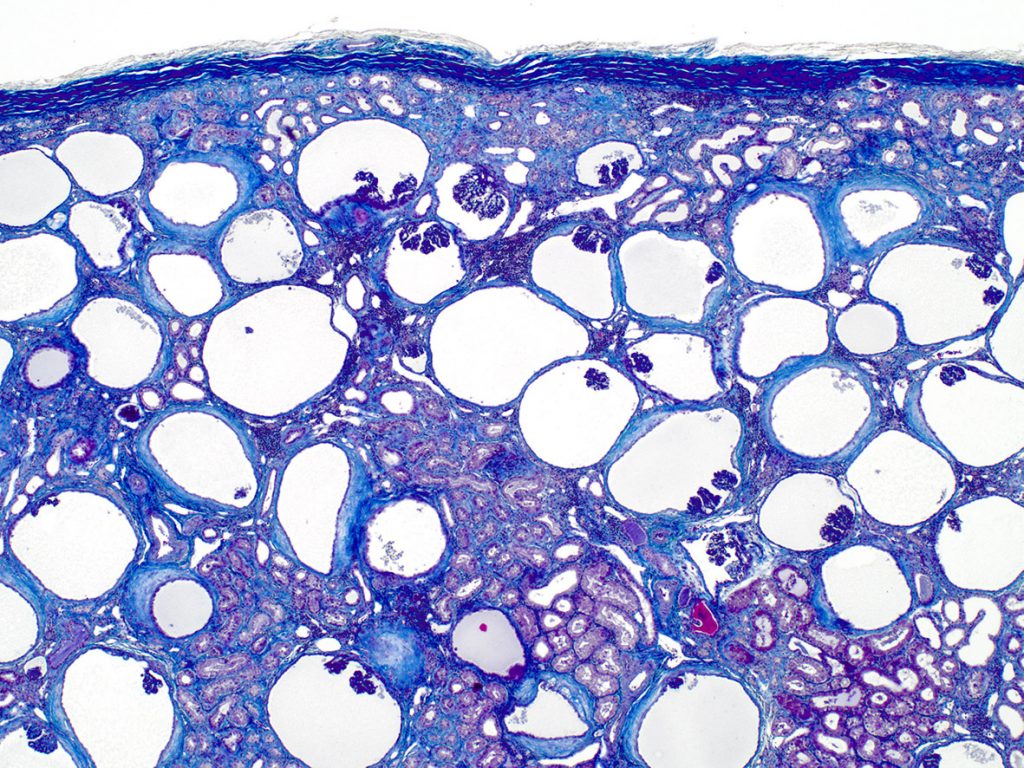
FIG.10C (MT): This stain highlights the collagen surrounding the dilated Bowman’s capsules and the mild peritubular fibrosis as well.
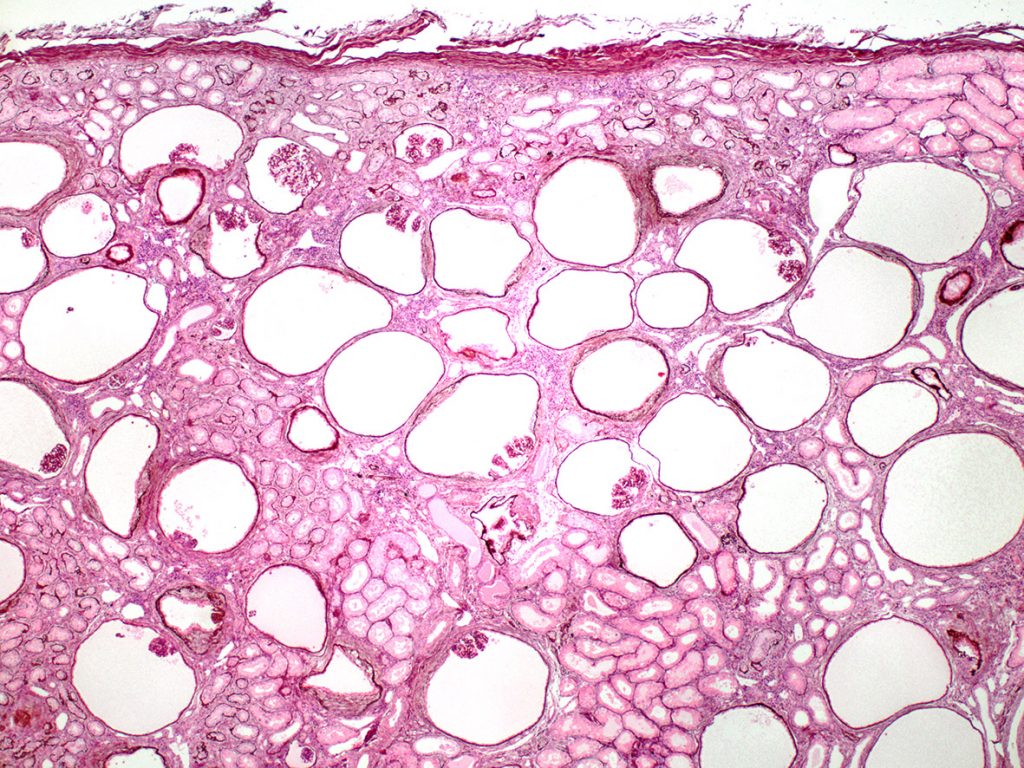
FIG.10D (PAMS): Similar to the PAS stain, the compressed glomerular tufts are easily identified with this stain.
Finally, if the maldevelopment involved a large proportion of nephrons, then the remaining “normal” nephrons undergo hypertrophy to compensate for the limited functioning nephron mass. However, more severe glomerular hypertrophy and hyperfiltration/glomerular hypertension may result in secondary focal segmental glomerulosclerosis (FSGS). Although this compensatory hypertrophy will also occur in older dogs that have lost nephrons (e.g. secondary to CKD), the hypertrophy is typically mild. In growing puppies, the glomeruli may become markedly to severely enlarged. Unfortunately, these glomerular changes can sometimes be mistakenly diagnosed as a “membranoproliferative (MPGN) pattern”. Given the association of MPGN and immune complex deposition, this type of misdiagnosis could lead to unwarranted immunosuppression. Therefore, large hypercellular glomeruli in young dogs should be alert the pathologist to look for other features of maldevelopment in order to avoid this type of mistake. Samples for TEM and IF can help with the final diagnoses in these confusing cases. Severe glomerular hypertrophy and hyperfiltration/glomerular hypertension may also result in secondary focal segmental glomerulosclerosis (FSGS).
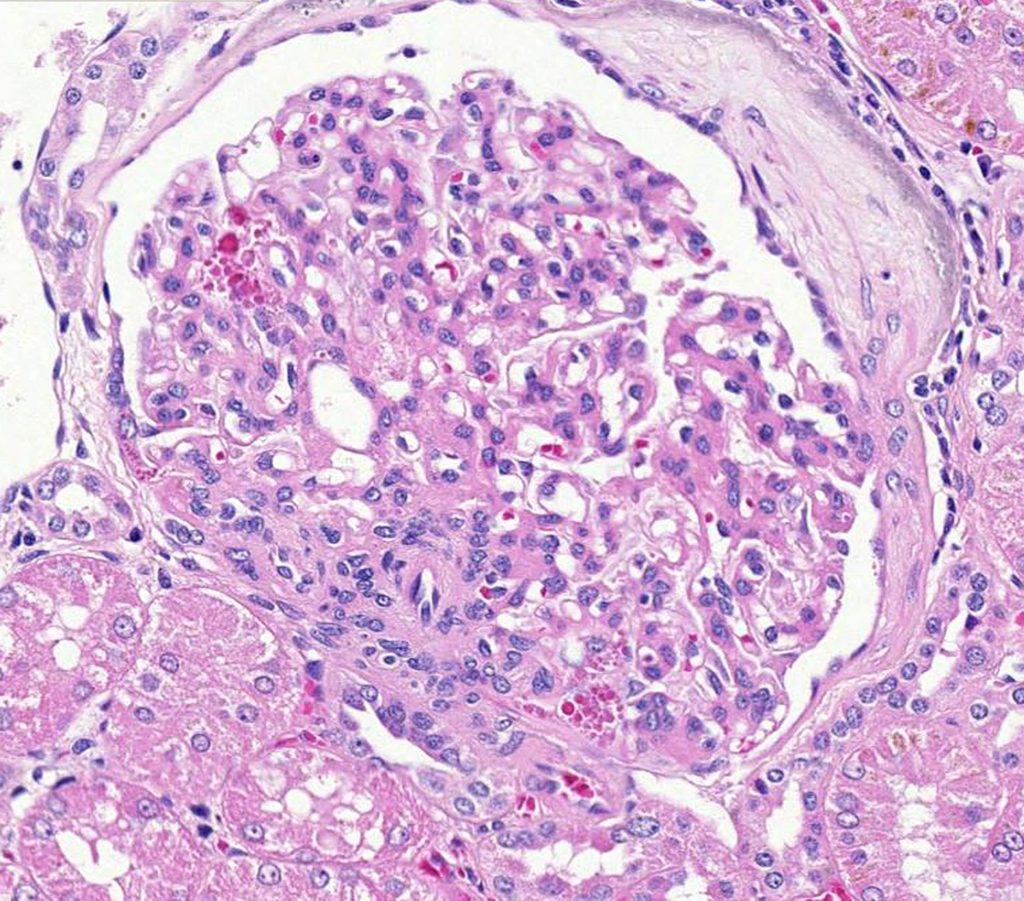
FIG.11A (HE): This glomerulus is markedly enlarged and the photomicrograph is 20X magnification, whereas most of the other glomeruli in this atlas were taken at 40X. There is a small synechiae and moderate periglomerular fibrosis. The podocytes contain promininent protein reabsorption droplets, indicative of injury to this cell lineage.
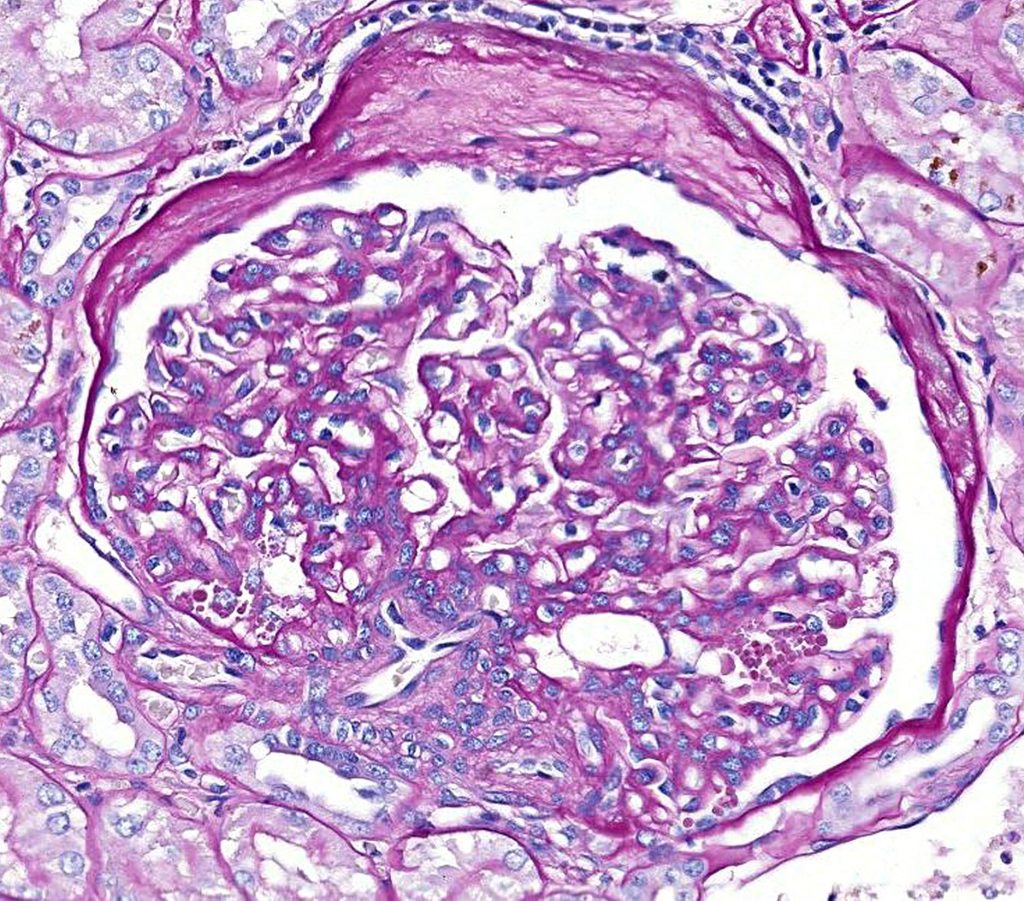
FIG.11B (PAS): Same glomerulus as depicted above. The synechia is not present in this section but there are prominent protein reabsorption droplets in podocytes.
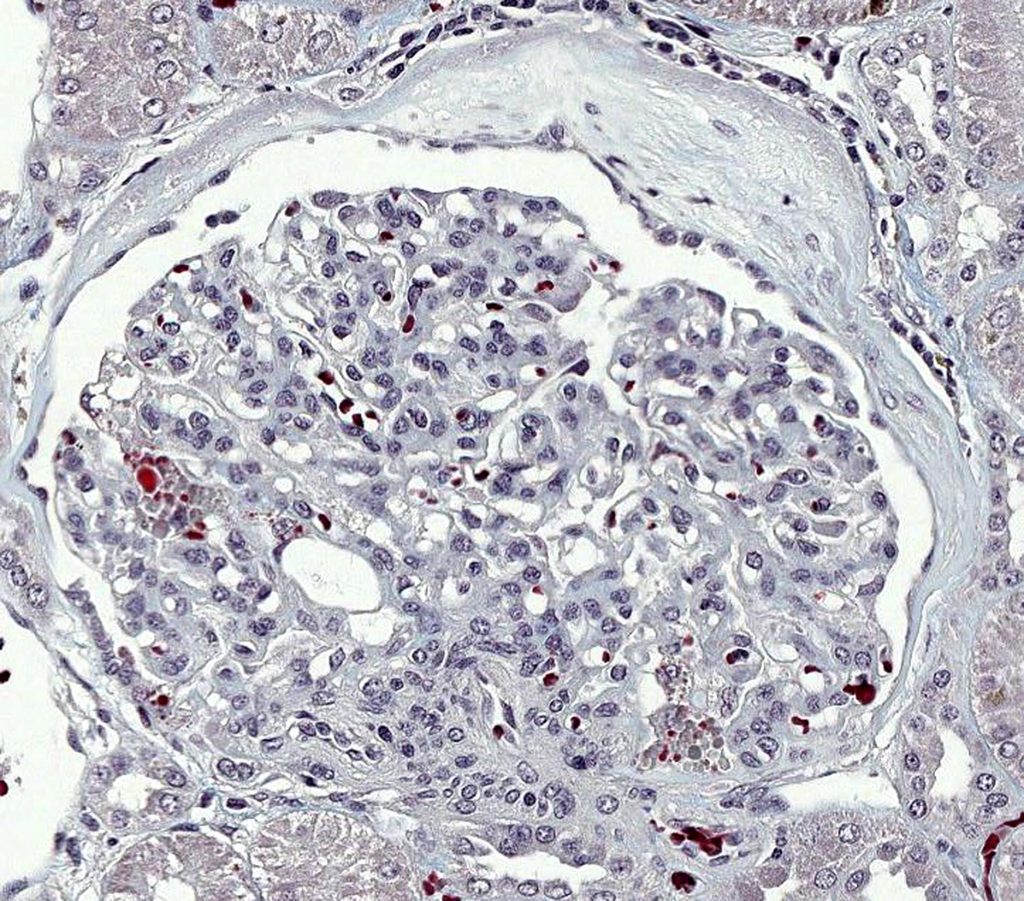
FIG.11C (MT): Same glomerulus as above. The podocyte protein droplets are bright red in this photomicrograph. (Please note that the blue collagen in this stain is paler than in other images because it was stained by a different histology laboratory than most of the other images.)
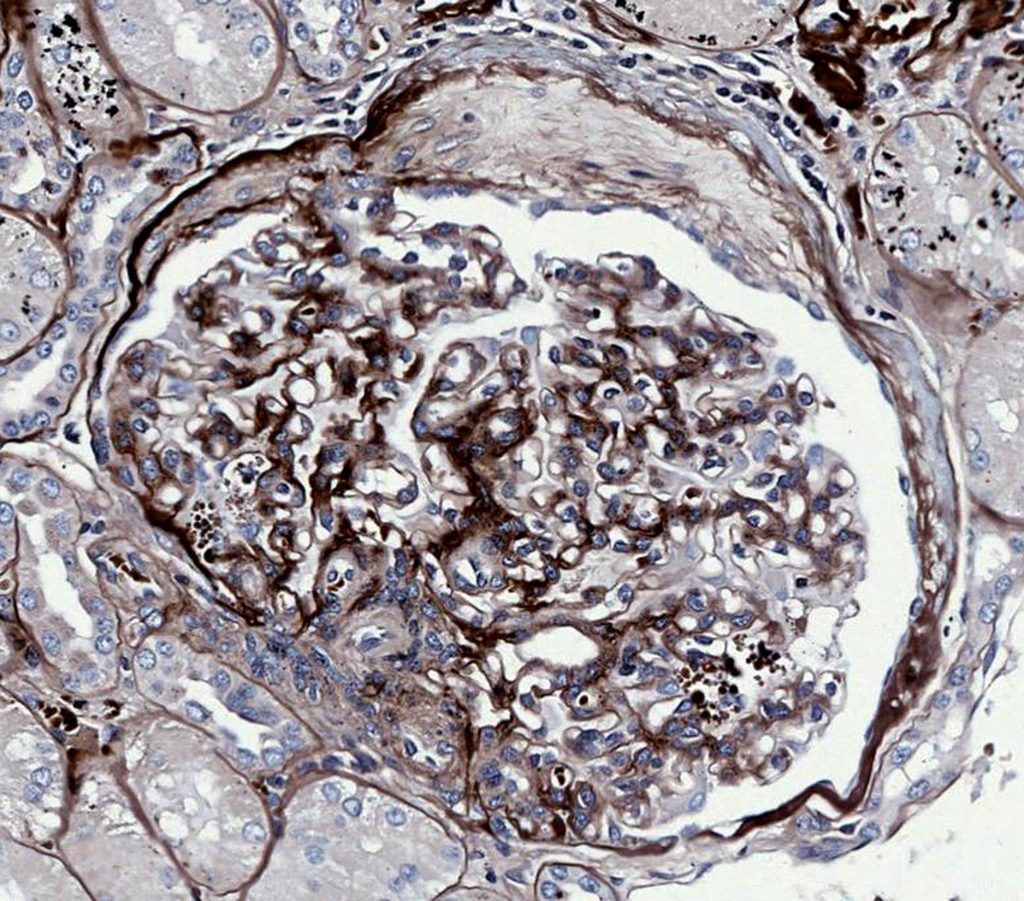
FIG.11D (JMS): Same glomerulus as above. There is another small synechia in a different location in this image.
Summary
As demonstrated above, there are a wide variety of chronic renal diseases that can be diagnosed in young dogs. Some diagnoses require TEM and, although they cannot yet be cured in dogs (or humans for that matter), a correct diagnosis is important for pedigree management and breeding decisions. Other diagnoses can be made based on histologic evaluation together with the clinical history (e.g. renal maldevelopment and ascending bacterial pyelonephritis). Clinicians are unlikely to submit a renal biopsy sample in order to diagnose pyelonephritis; however, diagnosing this condition in an autopsy sample from a neonate might be confusing because of the presence of fetal glomeruli. Moreover, correct diagnosis of maldevelopment (which could be hereditary) will help clinicians decide if relatives of the patient might be at risk. And to add one more layer of complexity, we have diagnosed young dogs with multiple of the previously described conditions. For example, dogs with malformed kidneys appear to be predisposed to urinary reflux and could develop ascending pyelonephritis. Additionally, although it is rare, we have diagnosed ICGN in young dogs that also had lesions consistent with renal maldevelopment.