Research Designs and Levels of Evidence
Partido, B.B.
Why do I need to know this?
Understanding types of research designs provides the foundation for applying evidence into clinical practice. It is critical to know what to search for, appraise the evidence, and determine whether the evidence can be applied in clinical practice.
Hierarchy of Research Designs 
Not all evidence is created equal. Most studies form the base of the hierarchy. As the studies progress higher up on the hierarchy, fewer studies exist.
Two categories of evidence sources exist: primary and secondary. The highest level of evidence is secondary evidence, which consists of Meta-Analysis and Systematic Reviews. The highest level of primary research is the Randomized Control Trial (RCT) (Level 1), followed by Cohort Studies (Level 2) and Case-Controlled Studies (Level 3). Case reports, case series, narrative reviews, and editorials (Levels 4 and 5) do not have a research design and do not fall into either category of primary or secondary evidence. Since animal and laboratory studies do not involve humans, they remain at the base of the hierarchy.
Secondary Evidence: Meta-Analysis
This involves statistical analysis that combines the results of multiple scientific studies. Many times, this type of analysis is part of a systematic review. By combining multiple studies, the power (generalization to larger population) of the results increases. The goal is to use this information to create Clinical Practice Guidelines (CPGs) which the Institute of Medicine defines as “systematically developed statements that assist practitioners with patient decisions about the most appropriate health care for specific clinical circumstances”.
Secondary Evidence: Systematic Review 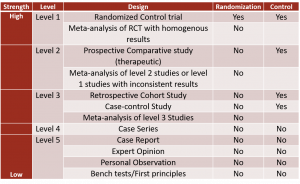
This involves a systematic process to collect primary sources, critically appraise the data, and synthesize the information. This reduces bias in the selection of the primary sources. The results of a systematic review provide the data for clinical guidelines.
Level 1- Primary Evidence: Randomized Control Trials (RCTs) 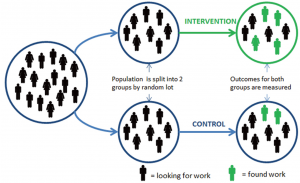
RCTs provide the strongest evidence for causal, (cause and effect) relationships. With these types of research designs, the following are included:
- At least 1 intervention (testing) treatment and 1 control treatment (placebo or no treatment).
- Participants are enrolled at the same time.
- Random assignment of participants into control and intervention groups.
- Measurements are take at multiple times, especially at the end to measure the outcomes of the treatments.
Primary Evidence: Quasi-Experimental Research Designs 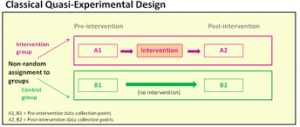
Quasi-experimental research designs provide evidence of relationships between variables but NOT causal relationships. In these studies, assignment of the groups is based on convenience and NOT random assignment.
Level 2- Primary Evidence: Cohort Studies 
Cohort studies are prospective (moving forward) studies that make observations about the association between an exposure or risk factor and the subsequent development of a disease or condition. Subjects can be grouped based on differences in exposure or risk factors. No one in the groups have the condition of interest but they are all followed over time to measure the development of the disease or condition.
Level 3- Primary Evidence: Case Control Studies 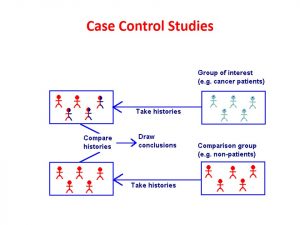
Case control studies are retrospective (looking backward) studies that make possible associations between a disease of interest and one or more risk factors. Investigators study subjects who have a certain disease or condition and compare them to control subjects who do not have the disease or condition. This is useful in studying the cause of rare diseases.
Level 4- Primary Evidence: Case report and case series
Case reports have no research design. They merely present the condition or treatment of an individual patient. A case series is when multiple case reports are presented. No statistical analysis can be made, therefore no statistical generalizations can be inferred to other patients.
Level 5- Primary Evidence: Narratives reviews, expert opinions, and editorials
These types of primary evidence are based on observations and opinions supported by the existing literature. They do not provide new knowledge.
Animal and Laboratory Studies
Since evidence based practice is dependent on the effect in humans, the lowest level of evidence is from animal and laboratory studies.