Research Ethics and the Institutional Review Board
Partido, B.B.
The protection of human subjects drives the purpose of the institutional review board (IRB) of any institution. The principal investigator, co-investigator(s), and key personnel must undergo specific Collaborative Institutional Training Initiative (CITI) training to ensure the safety of human subjects: Human Subjects Protection, Responsible Conduct of Research, and Signed Conflict of Interest Form.
The three core values, established from the Belmont Principles, are:
- Respect for persons: Treat people as autonomous
 individuals, protect those with diminished authority (i.e. informed consent process)
individuals, protect those with diminished authority (i.e. informed consent process) - Beneficence: Do good (beneficence) and do not harm (non-maleficence)
- Justice: Fairness of benefits and burdens
The IRB Approval Process
In order to submit a research study for IRB approval, the investigators must create a research protocol using the institution’s template, informed consent form using the institution’s template, and any additional forms. When all forms have been prepared, an application must be initiated and submitted into the IRB online system. The research study can fall into 1 of 3 categories:
- Exempt research– Does not need approval from the IRB because
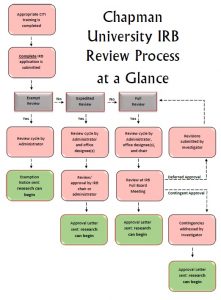 of limited contact with human subjects, use of de-identified data, and methods that involve minimal risk. (i.e. survey research studies)
of limited contact with human subjects, use of de-identified data, and methods that involve minimal risk. (i.e. survey research studies) - Expedited research– Contact with human subjects with minimal risks to the subjects (i.e. ergonomics study using photography). This application is reviewed by 1 member of the IRB.
- Convened board research– Contact with human subjects with more than minimal risks. (i.e. testing a new drug on human subjects). This required full review by the entire IRB, which meets once every 2 weeks.
Once the IRB grants approval, the investigators may begin the research study. If there are any any changes to the research study protocol or research team, an amendment must be submitted and approved by the IRB. If any incident occur during the study, an incident report must be submitted to the IRB.
Exempt research do not have an expiration date. For expedited and convened board research studies, the approval expires within 1 year. If the investigators have not completed the study or analyzing the data, an application for continuing review must be submitted. If the investigators have completed the study and do not need to access the data anymore, a final report must be submitted.