Skill Group: Dermatology
Goal Five: Fungal Examination and Culture
Before You Start …
Please refer to individual techniques for the equipment needed.
Introduction
Learning Outcomes
By the end of this lesson, you should be able to:
- Perform a Wood’s lamp examination and interpret the results.
- Explain how dermatophytes produce yellow-green florescence when exposed to UV light.
- Outline the steps to perform a Wood’s light examination.
- Explain the limitations of using a Wood’s light as the only diagnostic procedure for confirmation of a positive dermatophytosis diagnosis.
- Collect a fungal culture and interpret the results.
- Outline the progression of the findings you expect for a positive dermatophyte culture.
- Describe how a positive dermatophyte culture is confirmed.
- Identify clinical lesions that should be sampled for a fungal culture.
- Obtain samples for dermatophyte cultures.
Woods Lamp Examination: Technique
Wood’s lamp examination is a test that uses ultraviolet light (black light) emitted by the Wood’s lamp to closely examine hair and skin. Woods lamps are sometimes used as a screening test to detect dermatophytosis, but both the sensitivity and specificity of evaluation with a Wood’s lamp are quite low. When exposed to UV light, hairs invaded by Microsporum canis may result in a yellow-green fluorescence of the dermatophyte’s tryptophan metabolites in 30% to 80% of the isolates. The metabolites are only produced by dermatophytes that have invaded actively growing hairs.
Woods lamp examination is performed using a UV lamp and is a rapid and simple technique. Ideally, the patient would have not been bathed at least a day before the examination.
Make sure you have the following equipment: an ultraviolet light source that uses wavelengths of 320-450 nm (UV spectrum that does not damage vision or skin) that are filtered through a cobalt or nickel filters. The medical Wood’s lamp may include a variety of phosphors with different peak emission lengths, magnifying lens, white light or a black drape to exclude light. Avoid looking directly into the ultraviolet light!
Here are the steps for performing this technique:
- Turn on the lamp light and allow to warm up at least for 1-2 minutes so the appropriate wavelength can be produced.
- Darken the room. Allow your eyes to accommodate to the dark.
- Examine the skin and hair by holding the lamp within several inches (10-30 cm) away from the skin.
- Hairs should be exposed to the UV for at least 3 to 5 minutes as some dermatophyte strains are slow to show obvious apple-green/ yellow-green color.
- Infected hairs may be hidden beneath crusts; therefore, it is helpful to gently remove crusts prior to examination.
- All suspected dermatophyte infections should be confirmed by a concurrent fungal culture (dermatophyte test medium, DTM or Sabouraud dextrose agar).
Woods Lamp Examination: Interpretation

Fluorescence must be seen in individual hair shafts to be considered positive:
- If the short stubs of hair produce fluorescence, the proximal end of the hairs extracted from the follicles should also fluoresce. These fluorescing hairs should be plucked with forceps and used for inoculation of fungal medium or for microscopic examination.
- Early infections are characterized by fluorescence of the proximal portion of the hair shaft.
- In fully developed infections, the entire hair shaft glows.
- Other less common dermatophytes that may fluoresce include Microsporum distortum, Microsporum audouinii, and Trichophyton schoenleinii.
- Fluorescence is not present in scales or crusts or in dermatophyte cultures.
- Keratin, soap, petroleum, carpet fibers, and topical medications may fluoresce and give false positive reactions.
- Bacteria such as Pseudomonas aeruginosa and Corynebacterium minutissimum may fluoresce, but not with the apple-green color of a dermatophyte infected hair.
Dermatophyte Fungal Culture: Technique
A fungal culture is indicated in any pet with dermatitis that has a differential diagnosis of dermatophytosis. Performed appropriately, the fungal culture confirms the diagnosis and identifies the causative fungus. Fungal cultures are still considered the gold standard for diagnosing dermatophytes. Dermatophyte Test Medium (DTM) is a media used grow and isolate dermatophytes. Nitrogenous and carbonaceous compounds essential for microbial growth are provided by soy peptone. Dextrose serves as the energy source for metabolism. Chloramphenicol acts as a broad-spectrum antibiotic which inhibits a wide range of gram-positive and gram-negative bacteria. Cycloheximide is added to inhibit growth of saprophytic fungi. Phenol red, the pH indicator, is affected by the presence of dermatophytes (Microsporum, and Trichophyton spp.), which all produce alkaline metabolites. Production of alkali results in the medium changing from yellow-orange to red in color.
Dermatophyte fungal culture is performed by inoculation of the specimen on dermatophyte test medium or Sabouraud dextrose agar.
Make sure you have the following equipment: sterile hemostats or forceps, toothbrush, alcohol soaked 4×4 gauze sponge, sterile container or a new DTM slant vial, flask or biplate (SabDex + DTM), Wood’s lamp (optional)
Here are the steps for performing this technique; a video that walks through these steps is available:
- When Wood’s lamp examination is positive, hairs that fluoresce are selected and removed with sterile forceps or hemostats.
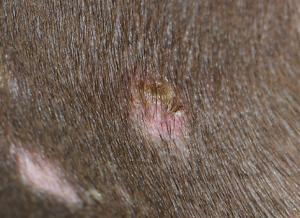
- Ideal collection sites are hair from the margins of the lesions, either newly formed or actively expanding lesions that have not been recently treated. Look for hairs that are broken or misshapen and associated with inflammation, scale or crust.
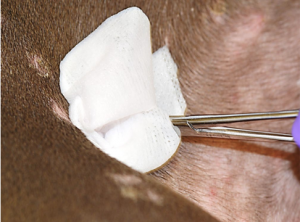
- Gently wipe the lesion using the sterile hemostats and a 70% alcohol-impregnated gauze or cotton and then letting it air dry for at least 2 minutes; this will decrease the growth of contaminants.
- Gently but forcibly pull the suspected infected hairs out of the follicles with the sterile hemostats or forceps in the direction that the hairs are growing.
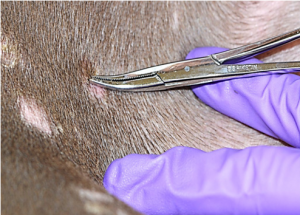
- Place the hair sample in a sterile container to be sent down to the OSU-VMC microbiology lab for culture, or a clean sterile container as specified by the laboratory where the sample will be sent. If the DTM culture is performed in house, directly inoculate the hairs into the media.
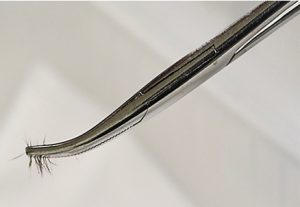

- Alternative method for detection of asymptomatic carriers: a sterile toothbrush is gently brushed through the animal’s coat to accumulate hair and keratin debris for at least a minute. The toothbrush is then gently pressed onto the surface of the DTM plate. This technique has proved especially valuable in detecting asymptomatic cats that may be carriers of M. canis.
- Fungal cultures submitted to the lab or kept-in house should be grown at least for 3 weeks. If fungal cultures are kept in-house:
- It is imperative that they be checked daily and findings recorded.
- Desiccation and exposure to UV light hinder growth.
- When bottles are used, it is also important to put the lid on loosely because bacterial contaminants grow faster in sealed jars.
- Cultures should be incubated in the dark at 30°C with 30% humidity for at least 10-21 days.
- Growing DTM cultures in house leads to a greater risk of clinic contamination and pose some risk to staff, therefore submission to a reference lab is recommended.
Dermatophyte Fungal Culture: Interpretation
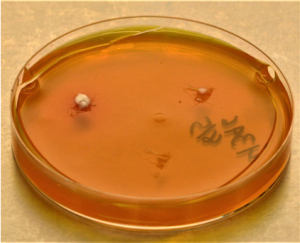
Dermatophytes first use protein in the medium, with alkaline metabolites turning the medium from yellow to red. When the protein is exhausted, the dermatophytes use carbohydrates, giving off acid metabolites, changing the media from red back to yellow. Colony growth is preceded by the focal color change in the media.
The timing of the color change is very important. Contaminant molds may also eventually use proteins after the carbohydrates sources run out, turning the media red once they are well established.
DTM cultures should be examined daily for the first 10 days. Generally, the colony has to grow for 7 to 10 days before macroconidia are produced. Sometimes, Trichophyton do not produce macroconidia.
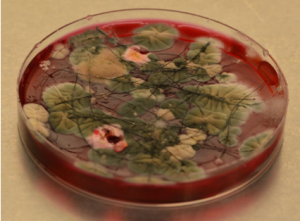
Dermatophyte colonies are always white, cream or tan. Contaminants are gray or green. Caution as bacteria and certain yeast can grow on this medium showing characteristic white or creamy bacteria like colonies. The plate in the following image has two white dermatophyte colonies and a large amount of contaminants.
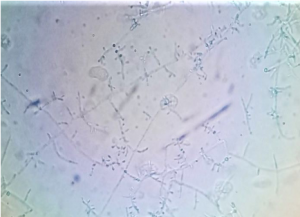
Collect macroconidia for microscopic examination, by applying the sticky side of clear cellophane tape to the aerial surface of the colony. The tape with the sample is then placed onto several drops of lactophenol cotton blue that is on the surface of a clean microscope slide. The slide can be examined under 100X.
False-positive results occur most commonly when the cultures are not observed frequently.
Fungi such as Blastomyces, Sporothrix, Histoplasmosis, Coccidioides and Pseudallescheria and some Aspergillus spp. may cause a change to red in DTM, so microscopic examination of the colony is essential to avoid an erroneous presumptive diagnosis.
Use of a Wood’s lamp examination and trichogram results can help confirm a positive fungal culture.
Wrapping Up
You are now able to select characteristics of a lesion that may represent a dermatophyte infection for which a Wood’s Lamp examination should be performed. You can explain the limitation of using a Wood’s Lamp for a confirmatory diagnosis of dermatophytosis. You can select a lesion and take a sample for submission of a fungal culture plated on dermatophyte test media. Furthermore, you can explain how to interpret the results of a DTM culture plate and make a slide to identify a potential dermatophyte. These skills will help you formulate differential diagnosis and treatment plan as well as explain the importance of asymptomatic carriers and zoonosis to pet owners.
Next, we will learn how to perform bacterial culture.
Before Moving On …
Use the self-check activities below to check your understanding.
(Under development)