Skill Group: Dermatology
Goal Three: Skin Scrapings
Before You Start …
Please refer to individual techniques for the equipment needed.
Introduction
Skin scrapes allow the clinician to find and identify small and microscopic ectoparasites. The technique used varies with the ectoparasite you are trying to identify. The sensitivity for ruling out a diagnosis depends on the disease and the aggressiveness of sampling. Although they are not always diagnostic, their relative ease and low cost make them essential test used in dermatology.
Learning Outcomes
By the end of this lesson, you should be able to:
- Collect deep and superficial skin scrapings as well as use other techniques to find parasites, and know the indications for each.
- List the ectoparasites that can be found by superficial skin scrapes and deep skin scrapes.
- Identify the optimal body locations to perform a superficial skin scrape to identify Sarcoptes scabiei.
- Differentiate between performing a superficial skin scrape and a deep skin scrape.
- Teach another student how to perform both a superficial and deep skin scrape using a #10 blade.
- Demonstrate two methods to collect surface/superficial ectoparasites and two methods to identify parasites that live within the hair follicles residing in the dermis.
Superficial Skin Scraping
Superficial skin scraping is performed using a new, sterile #10 blade. These are easily performed at most sites, however, scraping from sensitive areas around the face or feet may require sedation.
Surface mites that can be identified with this technique are Sarcoptes scabiei, Notoedres cati, Demodex gatoi, Trombicula alfreddugesi, Cheyletiella, and lice. In general, these mites can be difficult to find.
The goal is to collect surface scale, crust, and epidermal cells. Scrape skin that has not been excoriated. Preferable lesions include skin with erythema, raised papules and yellow or white crusts.
Make sure you have the following equipment: Clean glass slides, cover slips, new #10 surgical blade, mineral oil, pencil, microscope.
Here are the steps for performing this technique; a video that walks through these steps is available:
- Label a glass slide on the frosted area with the patient’s name and area of sample collection for example: “Millie” left ear.
- Place a large drop of mineral oil in the center of the labeled slide.

- Locate an area of alopecia, scale, crusts, hyperpigmentation, papule or erythema and apply a few drops of mineral oil to the area to be sampled. Applying mineral oil to the skin you are going to scrape as this helps trap the material for collection.
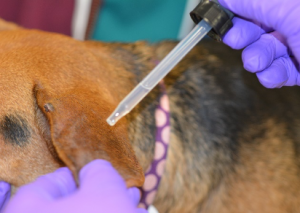
- Apply slight pressure to the skin surface to keep it from bunching up.
- Place and hold the flat portion of the blade on the skin at a right angle to the skin surface.
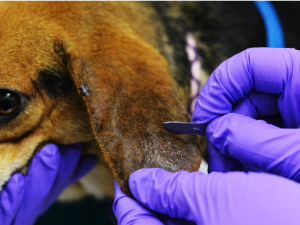
- Advance the blade in the same direction as the hair grows, using BROAD strokes to collect your sample from a large area, while you hold the skin taut. Scrape large areas and multiple areas to maximize the potential of collection. The more scrapings performed the more likely a diagnosis.
- Collect the sample of scale, crusts, epithelial cells and keratin debris in mineral oil the blade by using a scoping upward motion.
- For Sarcoptes scabiei, perform at least 5-10 scrapes, targeting the ear pinna margins, elbows, hocks, papular regions or areas of excessive crusts and scale.
- Remove all of the mineral oil and material from your blade by scraping the blade over the edge of the microscope slide. Move the material to the center of the slide into the mineral oil.
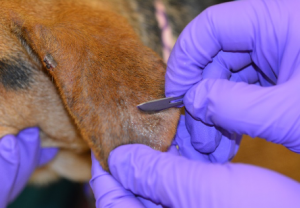
- Cover the slide with a cover slip directly over the area that you wish to examine under the microscope.
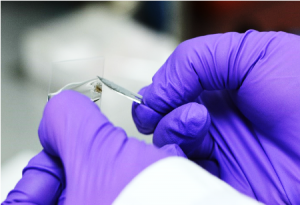


- Top tip! The coverslip should be floating evenly over the collected material in mineral oil with no visible air bubbles. If crusts are elevating the coverslip so that it is uneven, macerate the crusts and replace the coverslip. If the coverslip is uneven this makes microscopic examination very difficult as the material will require frequent re-focusing.
- Examine the slide under 4X or 10X with low light intensity and closure of the iris diaphragm on the condenser to provide increased contrast between mites and the mineral oil background.
A consistent orderly examination of the collected material should be done until a diagnosis is made or all the collected material has been examined. It is easiest to start the examination at one end of the scraped material mixed with oil and move the microscope stage straight across the slide in either horizontal or a vertical direction. At the end of the slide, the examination moves over one field of vision and goes back in the opposite direction. This is continued back and forth until all of the scraped material on the slide has been examined.
The #10 blade can be used to collect material from multiple sites as long as it is wiped clean between its uses at different sites.
Tape Impression
An alternative to superficial skin scrapings to find superficial ectoparasites that are considered surface dwellers such as Cheyletiella mites, Lynxacarus radovsky mites, Dermanyssus gallinae mites, and lice, is to press clear pressure sensitive adhesive tape to the hair surface and the skin adjacent to the parted hair or in shaved areas.
Make sure you have the following equipment: Clean glass slides, cover slips, new piece of clear pressure sensitive adhesive tape, microscope.
Here are the steps for performing this technique:
- Label a glass slide on the frosted area with the patient’s name and area of sample collection for example: “Fluffy” dorsal neck.
- Take a 3-4 cm piece of tape and press the sticky side down on the area of skin to be sampled. Press no more than two times to collect the hair and keratin material.
- Superficial scales, parasites and debris are collected. The tape is then stuck with pressure on a clean slide and examined with the microscope on 10X.
Deep skin scraping
Mites that live within the hair follicles can be obtained using this technique. The mites identified are all Demodex spp with the exception of Demodex gatoi which lives in the stratum corneum. In general, follicular mites are easy to find. The goal is to collect follicular Demodex from the hair follicles which reside in the dermis.
Deep skin scraping is performed using a new, sterile #10 blade. These are easily performed at most sites, however, scraping from sensitive areas around the face or feet may require sedation.
Make sure you have the following equipment: Clean glass slides, cover slips, new #10 surgical blade, mineral oil, pencil, microscope.
Here are the steps for performing this technique; a video that walks through these steps is available:
- Label a glass slide on the frosted area with the patient’s name and area of sample collection for example: “Fluffy” right thorax.
- Place a large drop of mineral oil in the center of the labeled slide.

- If the area to be sampled is covered with hair, it may be necessary to clip a small window to access the skin.
- Locate areas of alopecia, hypotrichosis, scale, crusts, hyperpigmentation, pustules, papules or erythema. Identify multiple sampling sites. If Demodex is on the differential list, at least 5 sites should be sampled.
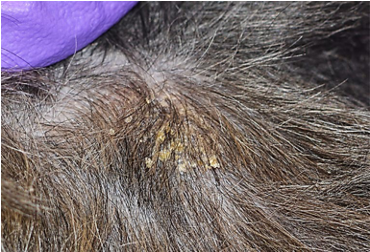
- The affected skin should be squeezed between the thumb and forefinger to help with extruding the mites from the hair follicles.
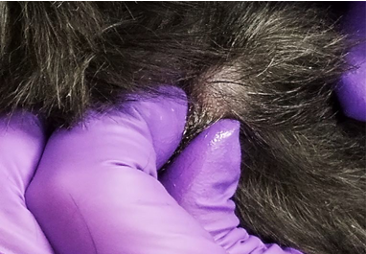
- Apply a drop of mineral oil to the area to be sampled or alternatively, to the surface of the blade. This will facilitate adherence of material to the blade.
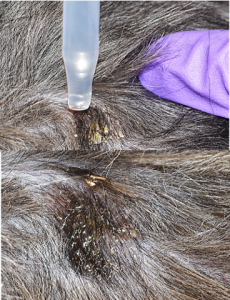
- The blade is held at an angle between 45 and 90 degrees to the skin surface, and moderate pressure is used to scrape the skin in the direction of hair growth.
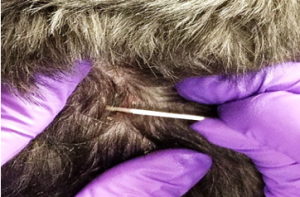
- Using short fast firm strokes, scrape the skin using your dominant hand, while ensuring to hold the skin taut with the thumb and index finger of your opposite hand to prevent bunching of the skin under the blade.
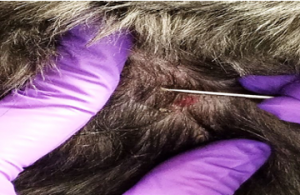
- The skin should appear pink, with the capillaries oozing small amounts of blood. This ensures that the material collected comes from deep enough within the skin to allow the collection of the follicular mites.
- The material is then scooped up on the blade, and placed on the slide by scraping the blade over the edge of the microscope slide. Move the material to the center of the slide into the mineral oil.

- Check to make certain you see blood droplets on the slide, if you do not, squeeze the skin again and repeat the scraping until capillary oozing is obtained.
- Place a coverslip on the material to be examined and ensure a uniform layer without air bubbles is achieved.


- Examine the slide under 10X with low light intensity and closure of the iris diaphragm on the condenser to provide increased contrast between mites and the mineral oil background.
A consistent orderly examination of the collected material should be done until a diagnosis is made or all the collected material has been examined. It is easiest to start the examination at one end of the scraped material mixed with oil and move the microscope stage straight across the slide in either horizontal or a vertical direction. At the end of the slide, the examination moves over one field of vision and goes back in the opposite direction. This is continued back and forth until all of the scraped material on the slide has been examined.
Record the site and the number of dead and live Demodex mites, as well as the presence of juvenile forms and eggs on the entire slide for each site sampled. This baseline count will allow assessment of improvement or lack thereof when the patient returns for re-evaluation after treatment has commenced. The same sites should be sampled on re-evaluation.
The #10 blade can be used to collect material from multiple sites as long as it is wiped clean between its uses at different sites.
Interpretation
For any of the surface mites, Sarcoptes scabiei, Notoedres cati, Demodex gatoi, Trombicula alfreddugesi, Otodectes, Cheyletiella, and lice, finding one parasite or egg is diagnostic! Often times, even with numerous scrapings, scabies is not found. It is only found in approximately 20-30% of the cases. If the disease is on the differential list a treatment trial is warranted. Despite the low yield of superficial skin scrapings for scabies, it is always better if you can provide a diagnosis due to the zoonotic aspect and associated implications.
For Demodex spp., scrapings taken from a normal dog, especially from the face, may contain an occasional adult mite. Although small number of mites may inhabit the skin of normal dogs, the probability of finding mites in normal dogs is very low. Multiple positive scrapings from different sites should be considered abnormal. Areas from which deep skin scrapes can be difficult to obtain diagnostic samples are regions of fibrosis, hyperplastic skin (e.g., interdigital, Shar-Pei) or firm nodules, therefore, consider performing trichograms and skin punch biopsies in these areas as additional useful tests. As a generalized Demodex infestation responds to treatment, the ratio of live to dead mites, decreases, as does the ratio of eggs and larvae to adults. If this is not occurring, the treatment regimen should be re- evaluated.
Cats often remove surface dwelling parasites by fastidious grooming. Fecal floatation can be used to find Cheyletiella and Demodex gatoi. When performing skin scrapes, take one from the dorsal neck, an area where cats have a more difficult time grooming.
| Mite | Diagnostic Test | Accuracy | Other Tests | Additional Tests |
|---|---|---|---|---|
| Demodex canis | Deep scrape | High | ||
| Demodex cati | Deep scrape | High | ||
| Demodex gatoi | Superficial scarpe | Low, mites often difficult to find | Possible identification of mites by fecal flotation | Response to fluralaner trial |
| Sarcoptes | Superficial scrape | Low (only 20%) | Response to treatment | Pinnal-pedal reflex (80%) |
| Otodectes | Otic mineral oil prep, superficial scrape | High | ||
| Cheyletiella | Flea comb, tape prep, superficial scrape | Moderate | Possible identification of mites by fecal flotation | |
| Lice |
Tape prep (can visualize) | High | ||
| Notoedres cati | Superficial scrape | High | ||
| Trombicula | Scrape of focal lesion | Moderate | ||
| Fleas | Flea comb | Moderate | Response to treatment | Rub flea feces “dirt” onto a moistened white paper towel, flea dirt will dissolve into a bloody smear |
Wrapping Up
You are now able to perform superficial and deep skin scrapes as well as to collect epidermal and follicular mites, respectively. You now understand which type of scrape is needed for each species of mite as you will know where the mite resides within the skin. You are now able to select lesions that should be sampled. Importantly, you now know how to prepare your slide and the microscope for sample reading. These skills will help you formulate differential diagnoses and treatment plans.
Next, we will learn how to perform trichogram.
Before Moving On …
Use the self-check activities below to check your understanding.
(Under development)